Page 148 - 2023 Taiwan Food and Drug Administration Annual Report
P. 148
2023 Taiwan Food and
Drug Administration
Annual Report
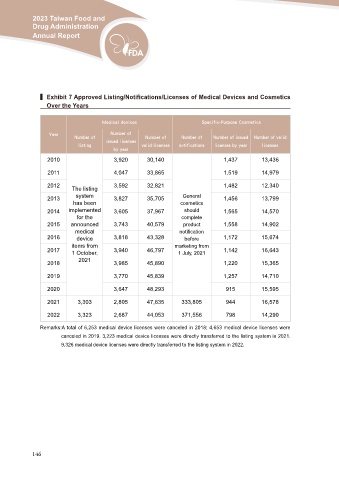
▍ Exhibit 7 Approved Listing/Notifications/Licenses of Medical Devices and Cosmetics
Over the Years
Medical devices Specific-Purpose Cosmetics
Year Number of Number of Number of Number of Number of issued Number of valid
listing issued licenses valid licenses
2010 notifications licenses by year licenses
by year
3,920 30,140 1,437 13,436
2011 4,047 33,865 1,519 14,979
2012 The listing 3,592 32,821 General 1,482 12,340
2013 system 3,827 35,705 cosmetics 1,456 13,799
2014 has been 3,605 37,967 1,565 14,570
2015 3,743 40,579 should 1,558 14,902
2016 implemented 3,818 43,328 complete 1,172 15,674
2017 for the 3,940 46,797 product 1,142 16,643
2018 3,985 45,890 notification 1,220 15,365
announced
medical before
device marketing from
items from 1 July, 2021
1 October,
2021
2019 3,770 45,839 1,257 14,710
2020 3,647 48,293 915 15,595
2021 3,303 2,805 47,635 333,805 944 16,578
2022 3,323 2,687 44,053 371,556 798 14,290
Remarks:A total of 6,253 medical device licenses were canceled in 2018; 4,653 medical device licenses were
canceled in 2019. 3,223 medical device licenses were directly transferred to the listing system in 2021.
9,326 medical device licenses were directly transferred to the listing system in 2022.
146