Page 147 - 2023 Taiwan Food and Drug Administration Annual Report
P. 147
7Appendix
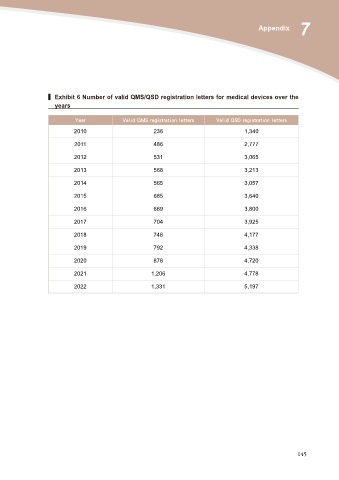
▍ Exhibit 6 Number of valid QMS/QSD registration letters for medical devices over the
years
Year Valid QMS registration letters Valid QSD registration letters
2010 236 1,340
2011 486 2,777
2012 531 3,065
2013 568 3,213
2014 565 3,057
2015 685 3,640
2016 669 3,800
2017 704 3,925
2018 748 4,177
2019 792 4,338
2020 878 4,720
2021 1,206 4,778
2022 1,331 5,197
145