Page 150 - 2023 Taiwan Food and Drug Administration Annual Report
P. 150
2023 Taiwan Food and
Drug Administration
Annual Report
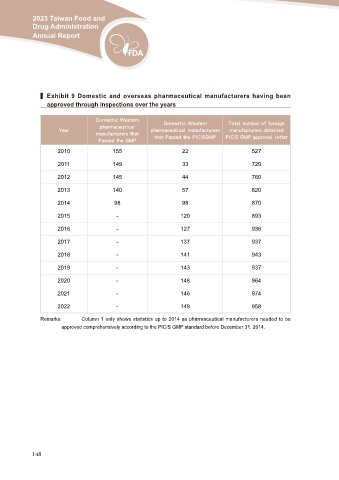
▍ Exhibit 9 Domestic and overseas pharmaceutical manufacturers having been
approved through inspections over the years
Domestic Western Domestic Western Total number of foreign
pharmaceutical
Year pharmaceutical manufacturers manufacturers obtained
2010 manufacturers that
Passed the GMP that Passed the PIC/SGMP PIC/S GMP approval letter
155 22 527
2011 149 33 720
2012 145 44 760
2013 140 57 820
2014 98 98 870
2015 - 120 893
2016 - 127 936
2017 - 137 937
2018 - 141 943
2019 - 143 937
2020 - 148 964
2021 - 146 974
2022 - 149 958
Remarks: Column 1 only shows statistics up to 2014 as pharmaceutical manufacturers needed to be
approved comprehensively according to the PIC/S GMP standard before December 31, 2014.
148