Page 153 - 2023 Taiwan Food and Drug Administration Annual Report
P. 153
7Appendix
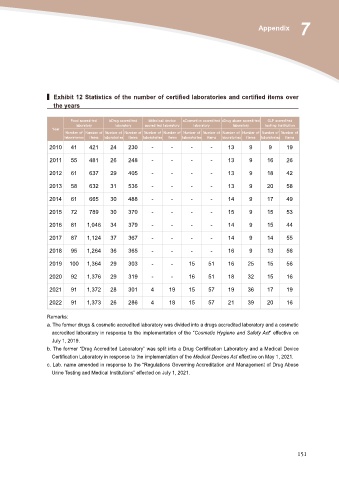
▍ Exhibit 12 Statistics of the number of certified laboratories and certified items over
the years
Food accredited bDrug accredited bMedical device aCosmetics accredited cDrug abuse accredited GLP accredited
Year laboratory laboratory accredited laboratory laboratory laboratory testing institution
Number of Number of Number of Number of Number of Number of Number of Number of Number of Number of Number of Number of
laboratories items laboratories items laboratories items laboratories items laboratories items laboratories items
2010 41 421 24 230 - - - - 13 9 9 19
2011 55 481 26 248 - - - - 13 9 16 26
2012 61 637 29 405 - - - - 13 9 18 42
2013 58 632 31 536 - - - - 13 9 20 58
2014 61 665 30 488 - - - - 14 9 17 49
2015 72 789 30 370 - - - - 15 9 15 53
2016 81 1,046 34 379 - - - - 14 9 15 44
2017 87 1,124 37 367 - - - - 14 9 14 55
2018 95 1,264 36 365 - - - - 16 9 13 56
2019 100 1,364 29 303 - - 15 51 16 25 15 56
2020 92 1,376 29 319 - - 16 51 18 32 15 16
2021 91 1,372 28 301 4 19 15 57 19 36 17 19
2022 91 1,373 26 286 4 18 15 57 21 39 20 16
Remarks:
a. The former drugs & cosmetic accredited laboratory was divided into a drugs accredited laboratory and a cosmetic
accredited laboratory in response to the implementation of the “Cosmetic Hygiene and Safety Act“ effective on
July 1, 2019.
b. The former “Drug Accredited Laboratory” was split into a Drug Certification Laboratory and a Medical Device
Certification Laboratory in response to the implementation of the Medical Devices Act effective on May 1, 2021.
c. Lab. name amended in response to the “Regulations Governing Accreditation and Management of Drug Abuse
Urine Testing and Medical Institutions” effected on July 1, 2021.
151