Page 134 - 2018食藥署年報(英文版)
P. 134
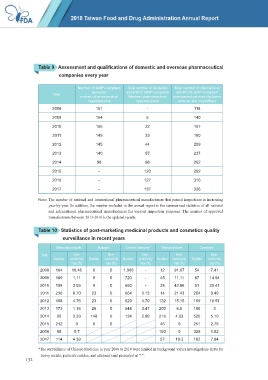
Table 9 Assessment and qualifications of domestic and overseas pharmaceutical
companies every year
Number of GMP-compliant Total number of domestic Total number of international
domestic and PIC/S GMP-compliant and PIC/S GMP-compliant
Year
modern pharmaceutical Western pharmaceutical pharmaceutical manufacturers
manufacturers manufacturers after on-site inspections
2008 151 - 118
2009 154 5 140
2010 155 22 157
2011 149 33 180
2012 145 44 209
2013 140 57 237
2014 98 98 262
2015 - 120 292
2016 - 127 316
2017 - 137 326
Note: The number of national and international pharmaceutical manufacturers that passed inspections is increasing
year-by-year. In addition, the number included in the annual report is the summarized statistics of all national
and international pharmaceutical manufacturers for various inspection purposes. The number of approved
manufacturers between 2013-2016 is the updated results.
Table 10 Statistics of post-marketing medicinal products and cosmetics quality
surveillance in recent years
Medicinal products Biologics Chinese Medicine* Medical devices Cosmetics
Year Non- Non- Non- Non- Non-
Number conformity Number conformity Number conformity Number conformity Number conformity
rate (%) rate (%) rate (%) rate (%) rate (%)
2008 164 16.46 0 0 1,000 - 12 91.67 54 7.41
2009 180 1.11 0 0 720 - 45 11.11 87 14.94
2010 198 3.03 0 0 660 - 28 42.86 51 29.41
2011 230 8.70 23 0 664 3.13 14 21.43 204 0.49
2012 168 4.76 23 0 629 4.70 132 15.15 109 16.51
2013 173 1.16 26 0 544 3.47 200 6.5 100 3
2014 90 3.33 148 0 134 2.99 216 4.63 520 5.19
2015 212 0 0 0 46 0 251 2.79
2016 88 5.7 193 0 329 1.52
2017 114 4.39 57 19.3 102 7.84
*The surveillance of Chinese Medicine in year 2008 to 2010 were limited in background values investigations (tests for
heavy metals, pesticide residue, and aflatozin) and presented as “-”.
132