Page 133 - 2018食藥署年報(英文版)
P. 133
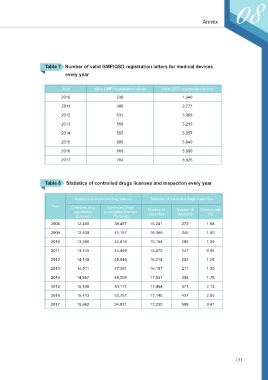
Table 7 Number of valid GMP/QSD registration letters for medical devices
every year
Year Valid GMP registration letters Valid QSD registration letters
2010 236 1,340
2011 486 2,777
2012 531 3,065
2013 568 3,213
2014 565 3,057
2015 685 3,640
2016 669 3,800
2017 704 3,925
Table 8 Statistics of controlled drugs licenses and inspection every year
Statistics of controlled drug licenses Statistics of controlled drugs inspection
Year Controlled drug Controlled drugs
registration prescription licenses Number of Number of Violation rate
(License) (Persons) inspection violations (%)
2008 12,465 39,467 16,241 270 1.66
2009 12,830 41,157 16,355 245 1.50
2010 13,266 42,619 15,154 196 1.29
2011 13,745 44,469 15,270 147 0.96
2012 14,149 45,844 16,214 202 1.25
2013 14,511 47,391 16,197 211 1.30
2014 14,857 49,059 17,057 304 1.78
2015 15,148 51,111 17,454 371 2.13
2016 15,413 52,757 17,145 437 2.55
2017 15,682 54,831 17,230 588 3.41
131