Page 129 - 2018食藥署年報(英文版)
P. 129
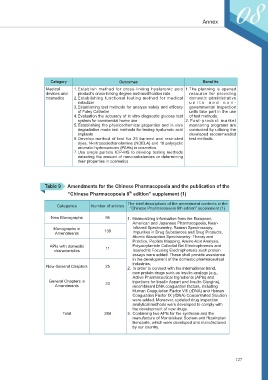
Category Outcomes Benefits
Medical 1. Establish method for cross-linking hyaluronic acid 1.The planning is opened
devices and product’s cross-linking degree and modification rate resource for providing
cosmetics 2. Establishing functional testing method for medical domestic administrative
nebulizer unit s and non-
3. Establishing test methods for analyze safety and efficacy governmental inspection
of Foley Catheter units take part in the use
4. Evaluation the accuracy of in vitro diagnostic glucose test of test methods.
system for commercial home use 2.Post-product market
5. Establishing the physicochemical properties and in vitro monitoring programs are
degradation mode test methods for testing hyaluronic acid conducted by utilizing the
implants developed recommended
6. Develop method of test for 25 banned and restricted test methods.
dyes, N-nitrosodiethanolamine (NDELA) and 18 polycyclic
aromatic hydrocarbons (PAHs) in cosmetics
7. Use single particle ICP-MS to develop testing methods
detecting the amount of nano-substances or determining
their properties in cosmetics
Table 9 Amendments for the Chinese Pharmacopoeia and the publication of the
th
“Chinese Pharmacopoeia 8 edition” supplement (1)
The brief descriptions of the amendment contents in the
Categories Number of articles
“Chinese Pharmacopoeia 8th edition” supplement (1)
New Monographs 95 1. Harmonizing information from the European,
American and Japanese Pharmacopoeia, Near-
Monographs in 130 Infrared Spectrometry, Raman Spectroscopy,
Amendments Impurities in Drug Substances and Drug Products,
Atomic Absorption Spectrometry: Theory and
Practice, Peptide Mapping, Amino Acid Analysis,
APIs with domestic 11 Polyacrylamide Colloidal Gel Electrophoresis and
characteristics Isoelectric Focusing Electrophoresis such protein
assays were added. These shall provide assistance
in the development of the domestic pharmaceutical
industries.
New General Chapters 25 2. In order to connect with the international trend,
new protein drugs such as insulin analogs (e.g.,
Active Pharmaceutical Ingredients (APIs) and
General Chapters in 23 injections for Insulin Aspart and Insulin Glargine),
Amendments recombinant DNA coagulation factors, including
Human Coagulation Factor VIII (rDNA) and Human
Coagulation Factor IX (rDNA) Concentrated Solution
were added. Moreover, updated drug inspection
analytical methods were developed to comply with
the development of new drugs.
Total 284 3. Containing two APIs for the synthesis and the
manufacture of Montelukast Sodium and Rizatriptan
Benzoate, which were developed and manufactured
by our country.
127