Page 140 - 2017食品藥物管理署年報(英文版)
P. 140
2017 Taiwan Food and Drug Administration Annual Report
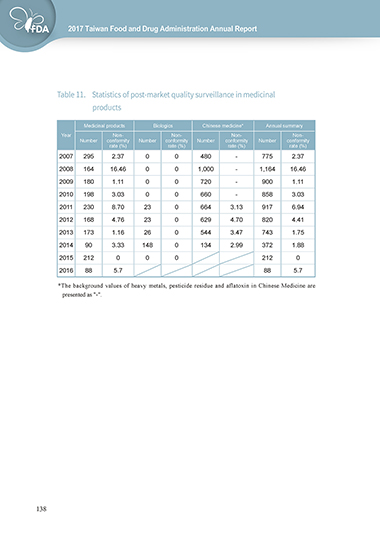
Table 11. Statistics of post-market quality surveillance in medicinal
products
Medicinal products Biologics Chinese medicine* Annual summary
Year Non- Non- Non- Non-
Number conformity Number conformity Number conformity Number conformity
rate (%) rate (%) rate (%) rate (%)
2007 295 2.37 0 0 480 - 775 2.37
2008 164 16.46 0 0 1,000 - 1,164 16.46
2009 180 1.11 0 0 720 - 900 1.11
2010 198 3.03 0 0 660 - 858 3.03
2011 230 8.70 23 0 664 3.13 917 6.94
2012 168 4.76 23 0 629 4.70 820 4.41
2013 173 1.16 26 0 544 3.47 743 1.75
2014 90 3.33 148 0 134 2.99 372 1.88
2015 212 0 0 0 212 0
2016 88 5.7 88 5.7
*The background values of heavy metals, pesticide residue and aflatoxin in Chinese Medicine are
presented as "-".
138