Page 145 - 2017食品藥物管理署年報(英文版)
P. 145
2017 Taiwan Food and Drug Administration Annual Report
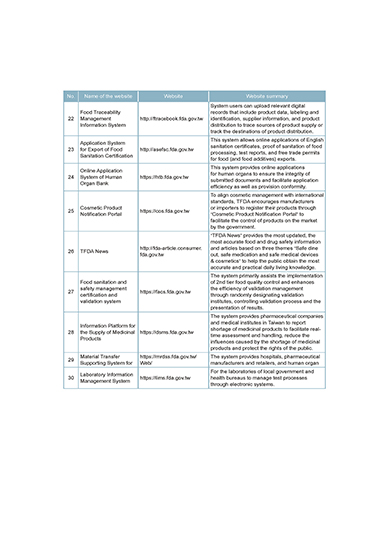
No. Name of the website Website Website summary
System users can upload relevant digital
Food Traceability records that include product data, labeling and
22 Management http://ftracebook.fda.gov.tw identification, supplier information, and product
Information System distribution to trace sources of product supply or
track the destinations of product distribution.
This system allows online applications of English
Application System
23 for Export of Food http://asefsc.fda.gov.tw sanitation certificates, proof of sanitation of food
processing, test reports, and free trade permits
Sanitation Certification
for food (and food additives) exports.
This system provides online applications
Online Application
24 System of Human https://htb.fda.gov.tw for human organs to ensure the integrity of
submitted documents and facilitate application
Organ Bank
efficiency as well as provision conformity.
To align cosmetic management with international
standards, TFDA encourages manufacturers
Cosmetic Product or importers to register their products through
25 https://cos.fda.gov.tw
Notification Portal “Cosmetic Product Notification Portal” to
facilitate the control of products on the market
by the government.
“TFDA News” provides the most updated, the
most accurate food and drug safety information
http://fda-article.consumer. and articles based on three themes “Safe dine
26 TFDA News
fda.gov.tw out, safe medication and safe medical devices
& cosmetics” to help the public obtain the most
accurate and practical daily living knowledge.
The system primarily assists the implementation
Food sanitation and of 2nd tier food quality control and enhances
safety management the efficiency of validation management
27 https://facs.fda.gov.tw
certification and through randomly designating validation
validation system institutes, controlling validation process and the
presentation of results.
The system provides pharmaceutical companies
and medical institutes in Taiwan to report
Information Platform for
28 the Supply of Medicinal https://dsms.fda.gov.tw shortage of medicinal products to facilitate real-
time assessment and handling, reduce the
Products
influences caused by the shortage of medicinal
products and protect the rights of the public.
Material Transfer https://mrdss.fda.gov.tw/ The system provides hospitals, pharmaceutical
29
Supporting System for Web/ manufacturers and retailers, and human organ
For the laboratories of local government and
Laboratory Information
30 https://lims.fda.gov.tw health bureaus to manage test processes
Management System
through electronic systems.