Page 141 - 2017食品藥物管理署年報(英文版)
P. 141
2017 Taiwan Food and Drug Administration Annual Report Annex
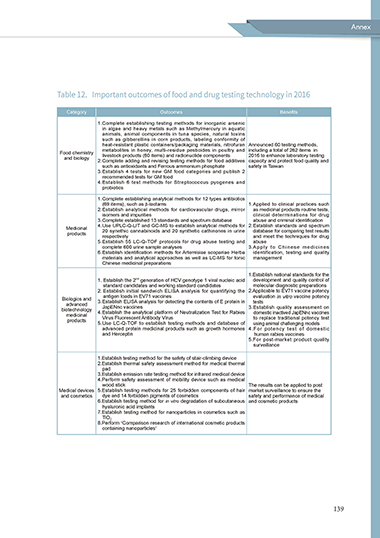
Table 12. Important outcomes of food and drug testing technology in 2016
Category Outcomes Benefits
1.Complete establishing testing methods for inorganic arsenic
in algae and heavy metals such as Methylmercury in aquatic
animals, animal components in tuna species, natural toxins
such as gibberellins in corn products, labeling conformity of
heat-resistant plastic containers/packaging materials, nitrofuran Announced 60 testing methods,
metabolites in honey, multi-residue pesticides in poultry and including a total of 262 items in
Food chemistry livestock products (60 items) and radionuclide components 2016 to enhance laboratory testing
and biology
2. Complete adding and revising testing methods for food additives capacity and protect food quality and
such as antioxidants and Ferrous ammonium phosphate safety in Taiwan
3. Establish 4 tests for new GM food categories and publish 2
recommended tests for GM food
4. Establish 6 test methods for Streptococcus pyogenes and
probiotics
1. Complete establishing analytical methods for 12 types antibiotics
(69 items), such as β-lactams 1. Applied to clinical practices such
2. Establish analytical methods for cardiovascular drugs, mirror as medicinal products routine tests,
isomers and impurities clinical determinations for drug
3. Complete established 13 standards and spectrum database abuse and criminal identification
Medicinal 4. Use UPLC-Q-LIT and GC-MS to establish analytical methods for 2. Establish standards and spectrum
products 20 synethic cannabinoids and 20 synthetic cathinones in urine database for comparing test results
respectively and meet the techniques for drug
5. Establish 55 LC-Q-TOF protocols for drug abuse testing and abuse
complete 600 urine sample analyses 3. Apply to Chinese medicines
6. Establish identification methods for Artemisiae scopariae Herba identification, testing and quality
materials and analytical approaches as well as LC-MS for tonic management
Chinese medicinal preparations
1.Establish national standards for the
nd
1. Establish the 2 generation of HCV genotype 1 viral nucleic acid development and quality control of
standard candidates and working standard candidates molecular diagnostic preparations
2. Establish initial sandwich ELISA analysis for quantifying the 2.Applicable to EV71 vaccine potency
antigen loads in EV71 vaccines evaluation in vitro vaccine potency
Biologics and 3. Establish ELISA analysis for detecting the contents of E protein in tests
advanced JapENnc vaccines
biotechnology 4. Establish the analytical platform of Neutralization Test for Rabies 3.Establish quality assessment on
domestic inactived JapENnc vaccines
medicinal Virus Fluorescent Antibody Virus to replace traditional potency test
products
5. Use LC-Q-TOF to establish testing methods and database of using animal challenging models
advanced protein medicinal products such as growth hormones 4.For potency test of domestic
and Herceptin human rabies vaccines
5.For post-market product quality
surveillance
1.Establish testing method for the safety of stair-climbing device
2.Establish thermal safety assessment method for medical thermal
pad
3.Establish emission rate testing method for infrared medical device
4.Perform safety assessment of mobility device such as medical
wood stick The results can be applied to post
Medical devices 5.Establish testing methods for 25 forbidden components of hair market surveillance to ensure the
and cosmetics dye and 14 forbidden pigments of cosmetics safety and performance of medical
6.Establish testing method for in vitro degradation of subcutaneous and cosmetic products
hyaluronic acid implants
7.Establish testing method for nanoparticles in cosmetics such as
TiO 2
8.Perform “Comparison research of international cosmetic products
containing nanoparticles”
139