Page 143 - 2017食品藥物管理署年報(英文版)
P. 143
2017 Taiwan Food and Drug Administration Annual Report Annex
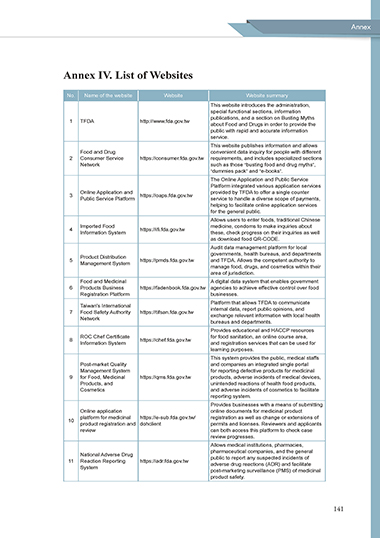
Annex IV. List of Websites
No. Name of the website Website Website summary
This website introduces the administration,
special functional sections, information
publications, and a section on Busting Myths
1 TFDA http://www.fda.gov.tw
about Food and Drugs in order to provide the
public with rapid and accurate information
service.
This website publishes information and allows
Food and Drug convenient data inquiry for people with different
2 Consumer Service https://consumer.fda.gov.tw requirements, and includes specialized sections
Network such as those “busting food and drug myths”,
“dummies pack” and “e-books”.
The Online Application and Public Service
Platform integrated various application services
Online Application and provided by TFDA to offer a single counter
3 https://oaps.fda.gov.tw
Public Service Platform service to handle a diverse scope of payments,
helping to facilitate online application services
for the general public.
Allows users to enter foods, traditional Chinese
Imported Food medicine, condoms to make inquiries about
4 https://ifi.fda.gov.tw
Information System these, check progress on their inquiries as well
as download food QR-CODE.
Audit data management platform for local
governments, health bureaus, and departments
Product Distribution
5 https://pmds.fda.gov.tw and TFDA. Allows the competent authority to
Management System
manage food, drugs, and cosmetics within their
area of jurisdiction.
Food and Medicinal A digital data system that enables government
6 Products Business https://fadenbook.fda.gov.tw agencies to achieve effective control over food
Registration Platform businesses.
Platform that allows TFDA to communicate
Taiwan's International
7 Food Safety Authority https://tifsan.fda.gov.tw internal data, report public opinions, and
exchange relevant information with local health
Network
bureaus and departments.
Provides educational and HACCP resources
ROC Chef Certificate for food sanitation, an online course area,
8 https://chef.fda.gov.tw
Information System and registration services that can be used for
learning purposes.
This system provides the public, medical staffs
Post-market Quality and companies an integrated single portal
Management System for reporting defective products for medicinal
9 for Food, Medicinal https://qms.fda.gov.tw products, adverse incidents of medical devices,
Products, and unintended reactions of health food products,
Cosmetics and adverse incidents of cosmetics to facilitate
reporting system.
Provides businesses with a means of submitting
Online application online documents for medicinal product
platform for medicinal https://e-sub.fda.gov.tw/ registration as well as change or extensions of
10
product registration and dohclient permits and licenses. Reviewers and applicants
review can both access this platform to check case
review progresses.
Allows medical institutions, pharmacies,
pharmaceutical companies, and the general
National Adverse Drug
11 Reaction Reporting https://adr.fda.gov.tw public to report any suspected incidents of
adverse drug reactions (ADR) and facilitate
System
post-marketing surveillance (PMS) of medicinal
product safety.
141