Page 134 - 2017食品藥物管理署年報(英文版)
P. 134
2017 Taiwan Food and Drug Administration Annual Report
Annex II. Important Outcomes and Statistics
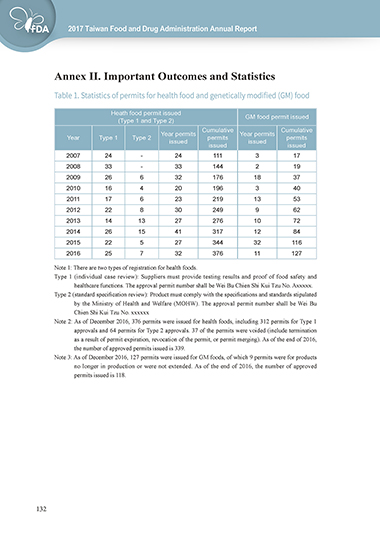
Table 1. Statistics of permits for health food and genetically modified (GM) food
Heath food permit issued GM food permit issued
(Type 1 and Type 2)
Cumulative Cumulative
Year permits Year permits
Year Type 1 Type 2 permits permits
issued issued
issued issued
2007 24 - 24 111 3 17
2008 33 - 33 144 2 19
2009 26 6 32 176 18 37
2010 16 4 20 196 3 40
2011 17 6 23 219 13 53
2012 22 8 30 249 9 62
2013 14 13 27 276 10 72
2014 26 15 41 317 12 84
2015 22 5 27 344 32 116
2016 25 7 32 376 11 127
Note 1: There are two types of registration for health foods.
Type 1 (individual case review): Suppliers must provide testing results and proof of food safety and
healthcare functions. The approval permit number shall be Wei Bu Chien Shi Kui Tzu No. Axxxxx.
Type 2 (standard specification review): Product must comply with the specifications and standards stipulated
by the Ministry of Health and Welfare (MOHW). The approval permit number shall be Wei Bu
Chien Shi Kui Tzu No. xxxxxx
Note 2: As of December 2016, 376 permits were issued for health foods, including 312 permits for Type 1
approvals and 64 permits for Type 2 approvals. 37 of the permits were voided (include termination
as a result of permit expiration, revocation of the permit, or permit merging). As of the end of 2016,
the number of approved permits issued is 339.
Note 3: As of December 2016, 127 permits were issued for GM foods, of which 9 permits were for products
no longer in production or were not extended. As of the end of 2016, the number of approved
permits issued is 118.
132