Page 131 - 2017食品藥物管理署年報(英文版)
P. 131
2017 Taiwan Food and Drug Administration Annual Report Annex
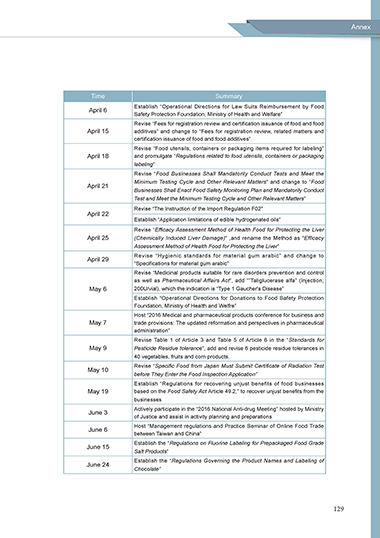
Time Summary
Establish “Operational Directions for Law Suits Reimbursement by Food
April 6
Safety Protection Foundation, Ministry of Health and Welfare”
Revise “Fees for registration review and certification issuance of food and food
April 15 additives” and change to “Fees for registration review, related matters and
certification issuance of food and food additives”
Revise “Food utensils, containers or packaging items required for labeling”
April 18 and promulgate “Regulations related to food utensils, containers or packaging
labeling”
Revise “Food Businesses Shall Mandatorily Conduct Tests and Meet the
Minimum Testing Cycle and Other Relevant Matters” and change to “Food
April 21
Businesses Shall Enact Food Safety Monitoring Plan and Mandatorily Conduct
Test and Meet the Minimum Testing Cycle and Other Relevant Matters”
Revise “The Instruction of the Import Regulation F02"
April 22
Establish “Application limitations of edible hydrogenated oils”
Revise “Efficacy Assessment Method of Health Food for Protecting the Liver
April 25 (Chemically Induced Liver Damage)" ,and rename the Method as "Efficacy
Assessment Method of Health Food for Protecting the Liver”
Revise “Hygienic standards for material gum arabic” and change to
April 29
“Specifications for material gum arabic”
Revise “Medicinal products suitable for rare disorders prevention and control
as well as Pharmaceutical Affairs Act”, add “”Taliglucerase alfa” (Injection;
May 6 200U/vial), which the indication is “Type 1 Gaucher's Disease”
Establish “Operational Directions for Donations to Food Safety Protection
Foundation, Ministry of Health and Welfre”
Host “2016 Medical and pharmaceutical products conference for business and
May 7 trade provisions: The updated reformation and perspectives in pharmaceutical
administration”
Revise Table 1 of Article 3 and Table 5 of Article 6 in the “Standards for
May 9 Pesticide Residue tolerance”, add and revise 6 pesticide residue tolerances in
40 vegetables, fruits and corn products.
Revise “Specific Food from Japan Must Submit Certificate of Radiation Test
May 10
before They Enter the Food Inspection Application”
Establish “Regulations for recovering unjust benefits of food businesses
May 19 based on the Food Safety Act Article 49.2,” to recover unjust benefits from the
businesses
Actively participate in the “2016 National Anti-drug Meeting” hosted by Ministry
June 3
of Justice and assist in activity planning and preparations
Host “Management regulations and Practice Seminar of Online Food Trade
June 6
between Taiwan and China”
Establish the “Regulations on Fluorine Labeling for Prepackaged Food Grade
June 15
Salt Products”
Establish the “Regulations Governing the Product Names and Labeling of
June 24
Chocolate”
129