Page 130 - 2017食品藥物管理署年報(英文版)
P. 130
2017 Taiwan Food and Drug Administration Annual Report
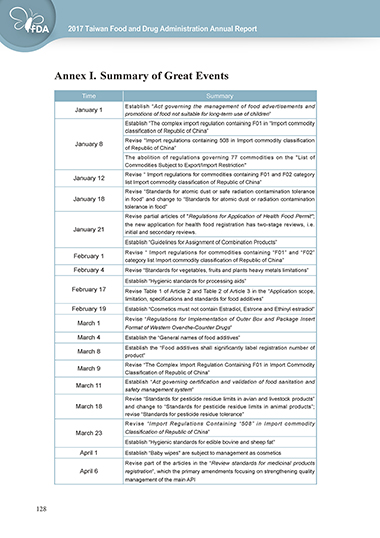
Annex I. Summary of Great Events
Time Summary
Establish “Act governing the management of food advertisements and
January 1
promotions of food not suitable for long-term use of children”
Establish “The complex import regulation containing F01 in “Import commodity
classification of Republic of China”
Revise “Import regulations containing 508 in Import commodity classification
January 8
of Republic of China”
The abolition of regulations governing 77 commodities on the "List of
Commodities Subject to Export/Import Restriction"
Revise “ Import regulations for commodities containing F01 and F02 category
January 12
list Import commodity classification of Republic of China”
Revise “Standards for atomic dust or safe radiation contamination tolerance
January 18 in food” and change to “Standards for atomic dust or radiation contamination
tolerance in food”
Revise partial articles of "Regulations for Application of Health Food Permit";
the new application for health food registration has two-stage reviews, i.e.
January 21 initial and secondary reviews.
Establish “Guidelines for Assignment of Combination Products”
Revise “ Import regulations for commodities containing “F01” and “F02”
February 1
category list Import commodity classification of Republic of China”
February 4 Revise “Standards for vegetables, fruits and plants heavy metals limitations”
Establish “Hygienic standards for processing aids”
February 17 Revise Table 1 of Article 2 and Table 2 of Article 3 in the “Application scope,
limitation, specifications and standards for food additives”
February 19 Establish “Cosmetics must not contain Estradiol, Estrone and Ethinyl estradiol”
Revise “Regulations for Implementation of Outer Box and Package Insert
March 1
Format of Western Over-the-Counter Drugs”
March 4 Establish the “General names of food additives”
Establish the “Food additives shall significantly label registration number of
March 8
product”
Revise “The Complex Import Regulation Containing F01 in Import Commodity
March 9
Classification of Republic of China”
Establish “Act governing certification and validation of food sanitation and
March 11
safety management system”
Revise “Standards for pesticide residue limits in avian and livestock products”
March 18 and change to “Standards for pesticide residue limits in animal products”;
revise “Standards for pesticide residue tolerance”
Revise “Import Regulations Containing “508” in Import commodity
March 23 Classification of Republic of China”
Establish “Hygienic standards for edible bovine and sheep fat”
April 1 Establish “Baby wipes" are subject to management as cosmetics
Revise part of the articles in the “Review standards for medicinal products
April 6 registration”, which the primary amendments focusing on strengthening quality
management of the main API
128