Page 132 - 2017食品藥物管理署年報(英文版)
P. 132
2017 Taiwan Food and Drug Administration Annual Report
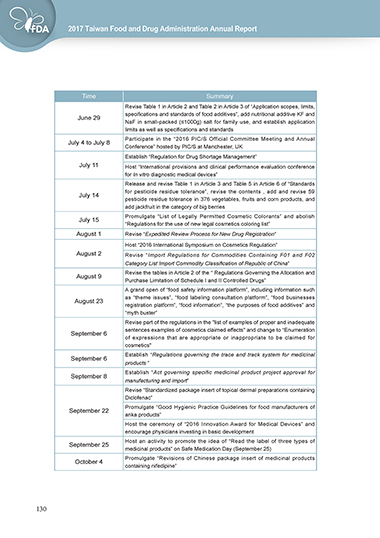
Time Summary
Revise Table 1 in Article 2 and Table 2 in Article 3 of “Application scopes, limits,
specifications and standards of food additives”, add nutritional additive KF and
June 29
NaF in small-packed (≤1000g) salt for family use, and establish application
limits as well as specifications and standards
Participate in the “2016 PIC/S Official Committee Meeting and Annual
July 4 to July 8
Conference” hosted by PIC/S at Manchester, UK
Establish “Regulation for Drug Shortage Management”
July 11 Host “International provisions and clinical performance evaluation conference
for In vitro diagnostic medical devices”
Release and revise Table 1 in Article 3 and Table 5 in Article 6 of “Standards
for pesticide residue tolerance”, revise the contents , add and revise 59
July 14
pesticide residue tolerance in 376 vegetables, fruits and corn products, and
add jackfruit in the category of big berries
Promulgate “List of Legally Permitted Cosmetic Colorants” and abolish
July 15
“Regulations for the use of new legal cosmetics coloring list”
August 1 Revise “Expedited Review Process for New Drug Registration”
Host “2016 International Symposium on Cosmetics Regulation”
August 2 Revise “Import Regulations for Commodities Containing F01 and F02
Category List Import Commodity Classification of Republic of China”
Revise the tables in Article 2 of the “ Regulations Governing the Allocation and
August 9
Purchase Limitation of Schedule I and II Controlled Drugs”
A grand open of “food safety information platform”, including information such
as “theme issues”, “food labeling consultation platform”, “food businesses
August 23
registration platform”, “food information”, “the purposes of food additives” and
“myth buster”
Revise part of the regulations in the "list of examples of proper and inadequate
sentences examples of cosmetics claimed effects" and change to “Enumeration
September 6
of expressions that are appropriate or inappropriate to be claimed for
cosmetics"
Establish “Regulations governing the trace and track system for medicinal
September 6
products ”
Establish “Act governing specific medicinal product project approval for
September 8
manufacturing and import”
Revise “Standardized package insert of topical dermal preparations containing
Diclofenac”
Promulgate “Good Hygienic Practice Guidelines for food manufacturers of
September 22
anka products”
Host the ceremony of “2016 Innovation Award for Medical Devices” and
encourage physicians investing in basic development
Host an activity to promote the idea of “Read the label of three types of
September 25
medicinal products” on Safe Medication Day (September 25)
Promulgate “Revisions of Chinese package insert of medicinal products
October 4
containing nifedipine”
130