Page 126 - 2017食品藥物管理署年報(英文版)
P. 126
2017 Taiwan Food and Drug Administration Annual Report
for managing product quality assurance, factory management, distribution, and
auditing. An additional five administrative offices were also established to support
TFDA's administration and management, namely: Office of the Secretariat, Office of
Personnel, Office of Service Ethics, Office of Accounting, and Office of Information
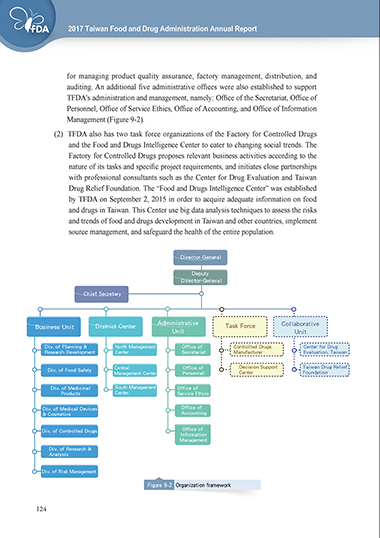
Management (Figure 9-2).
(2) TFDA also has two task force organizations of the Factory for Controlled Drugs
and the Food and Drugs Intelligence Center to cater to changing social trends. The
Factory for Controlled Drugs proposes relevant business activities according to the
nature of its tasks and specific project requirements, and initiates close partnerships
with professional consultants such as the Center for Drug Evaluation and Taiwan
Drug Relief Foundation. The “Food and Drugs Intelligence Center” was established
by TFDA on September 2, 2015 in order to acquire adequate information on food
and drugs in Taiwan. This Center use big data analysis techniques to assess the risks
and trends of food and drugs development in Taiwan and other countries, implement
source management, and safeguard the health of the entire population.
Director-General
Deputy
Director-General
Chief Secretary
Business Unit District Center Administrative Task Force Collaborative
Unit Unit
Div. of Planning & North Management Office of Controlled Drugs Center for Drug
Research Development Center Secretariat Manufacturer Evaluation, Taiwan
Div. of Food Safety Central Office of Decision Support Taiwan Drug Relief
Management Center Personnel Center Foundation
Div. of Medicinal South Management Office of
Products Center Service Ethics
Div. of Medical Devices Office of
& Cosmetics Accounting
Div. of Controlled Drugs Office of
Information
Management
Div. of Research &
Analysis
Div. of Risk Management
Figure 9-2 Organization framework
124