
Food and Drug Administration
56
Policies and Outcomes
1. Creating the Legal Environment and Harmonization with International Standards
(1) Drafting the Revised Statute for Control of Cosmetic Hygiene
a. To establish a legal environment harmonized with global cosmetic management standards, TFDA
is referencing international specifications to revise definitions for cosmetics and replace existing
inspection and registration systems for medicated cosmetics and cosmetic colorants with the
Product Information File (PIF) for the premarket product registration and management system.
Source management will also be strengthened, with plans made for implementing border sample
inspections and implementation of a GMP system for all relevant cosmetics. Cosmetic advertisement
management models have also been revised to phase out the preliminary examination system of
cosmetic advertisements. Minimum limits to fines have been added, and amounts the maximum
limits of fines have been raised. New rules have also been introduced defining adverse reactions to
cosmetics that must be reported as well as withdrawal or recalls of nonconforming cosmetics by
cosmetic companies.
b. In 2014, revisions to the
Statute for Control of Cosmetic Hygiene
have been drafted. A total of four
corporate impact forums, three cosmetics registration and system operation seminars, and three
cosmetic GMP rules seminars were held to collect public opinions and improve awareness for the
relevant policies.
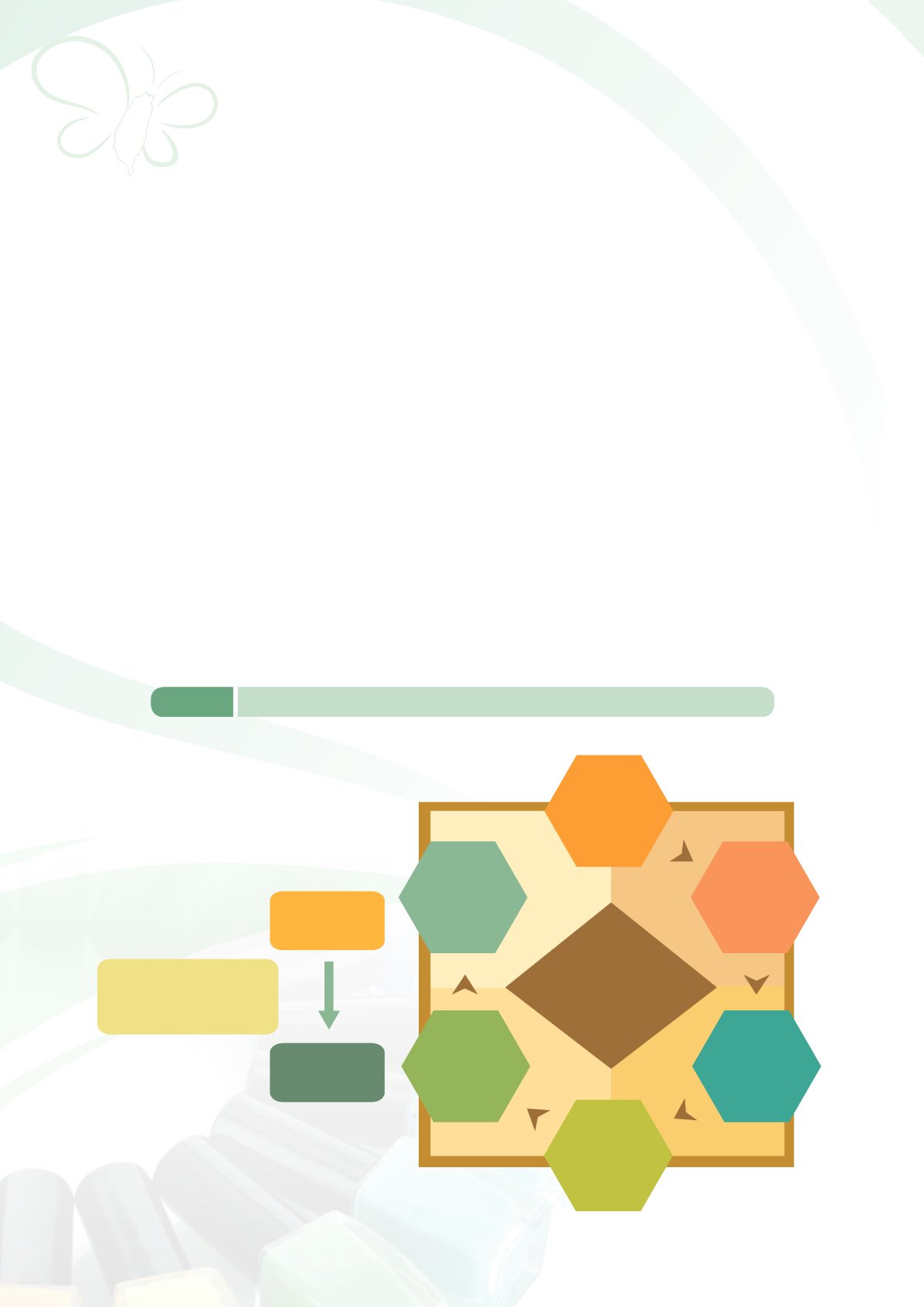
Elevating cosmetic
management regulations
from
Statute to Act.
Statute
for
Control of Cosmetic
Hygiene
Added
ADR reporting
Border inspection
Rules on
product withdrawal
and recalls
Temporarily
retained
Inspectionand
registrationof specific
products (during the
provisional period)
Eliminating
criminal sentencing
Increasing fines
Added
Whistleblower
clause
s
Added
Product Registration
PIF registration
Complete GMP
implementation
Eliminated
Preliminary
examination of
advertise ments
Inspection and
registration of
colorants
Placing greater importance
on corporatemanagement
responsibility
Heavy punishments for
willful violators
Changes
Definition of
cosmetics
Act for Control of
Cosmetic Hygiene
Figure 6-2
Key revisions to the overall
Statute for Control of Cosmetic Hygiene



















