
Food and Drug Administration
58
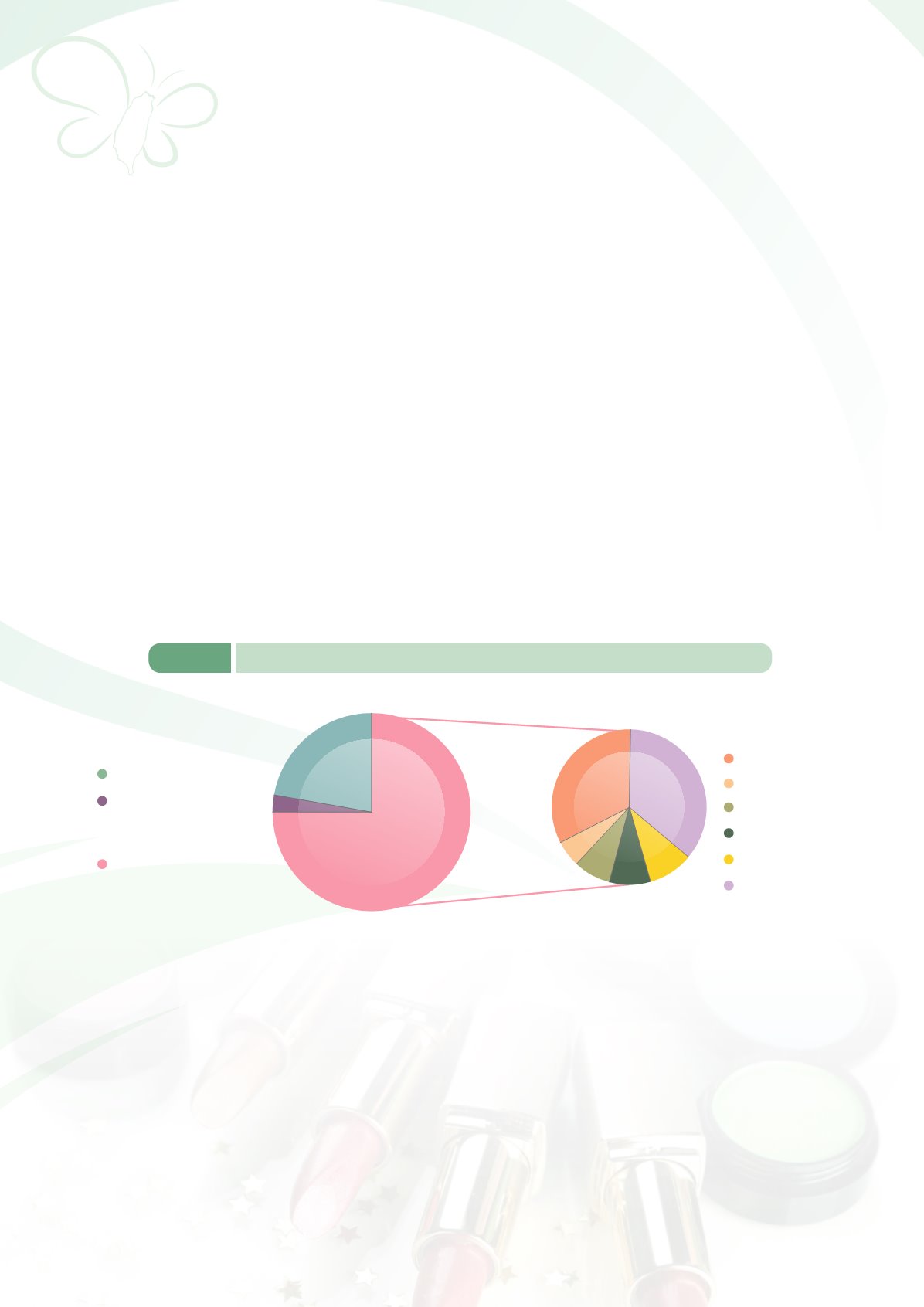
b. TFDA approved and issued a total of 28,708 licenses in 2014, of which 6,219 licenses were
granted to domestically made cosmetics while 22,489 licenses were granted to imported products
(Figure 6-3).
(2) Cosmetic Advertisement Examination
a. To help business owners'gain better understanding on the procedures for producing and applying
for cosmetic advertisements, TFDA has promulgated the
Guidelines for Cosmetics Advertising
and Rules for Application of Drugs and Cosmetics Advertising for reference and compliance.
Additionally, TFDA has produced leaflets titled Tips for Identifying Legal Cosmetic Advertisements
to help consumers correctly identify legal advertisements.
b. To unify examination standards for cosmetic advertisements, TFDA has promulgated the
Cosmetics
Advertising Act and Examination Manual
and an
Enumeration of Expressions that are Appropriate or
Inappropriate to be Claimed for Cosmetics.
To ensure the comprehensiveness of rules governing the
examination of cosmetic advertisements, TFDA is also using its cosmetic advertisement management
consultation committee to investigate special cases and provide professional opinions.
c. In 2014, TFDA received a total of 1,449 applications for cosmetic advertisements, of which 1,286
were approved (88.7%).
Japan 32%
Italy 12%
US 11%
Others 28%
Germany 7%
France 10%
Imported from
Mainland China
932 (3%)
Imported 21557 (75%)
Domestic 6219 (22%)
Imported from
Figure 6-3
Number of licenses granted for medicated cosmetics as of 2014



















