
2015 Annual Report
59
Risk Assessment
Management and
Research Outcomes
Cosmetics
Management
Appendix
National
Laboratory and
Testing Network
Risk Communication
and Consumer
Protection
International
Cooperation and
Cross-Strait Exchange
Food
Management
Medicinal
Products
Management
Policy and
Organization
Controlled
Drugs
Management
Medical
Devices
Management
Section 2 Cosmetics Source Management
Current Status
Since 2008, TFDA has been working with the Industrial Development Bureau (IDB) of the Ministry of
Economic Affairs (MOEA) to promote voluntary compliance with GMP among cosmetics manufacturers
in order to ensure the quality of manufactured products. Currently, cosmetics manufacturers are required
to submit documented review information to the IDB. An audit team established by the IDB conducts
the audit. Manufacturers who have passed the audit may then apply for a GMP certificate from TFDA. To
conform with international GMP for cosmetics and greatly improve the manufacturing standards of the
cosmetic industry in Taiwan, TFDA and the IDB have also amended the
Enforcement Focuses for Voluntary
Compliance to GMP Standards
on 9 June 2014, and established Taiwan's national standard CNS 22716
as the reference for verifying the quality management system involved in order to align the management
system to the international standard ISO 22716. Suppliers can refer to the state and requirements of
actual production as well as dosage form or product to define the scope of their certificate applications.
Cosmetic management strategies and measures between countries have also been referenced to amend
the
Statute for Control of Cosmetic Hygiene
and establish a new section of
Cosmetics Product Notification
Portal
. Business owners are encouraged to voluntarily register themselves at the system's portal. Once
the revisions have been passed, registration will become mandatory in order to eliminate the need for pre-
market registration, shorten the time-to-market for the products, and provide TFDA with full knowledge of
all cosmetics currently being marketed for the purpose of providing consumers with effective protection.
Policies and Outcomes
1. Production Source Control
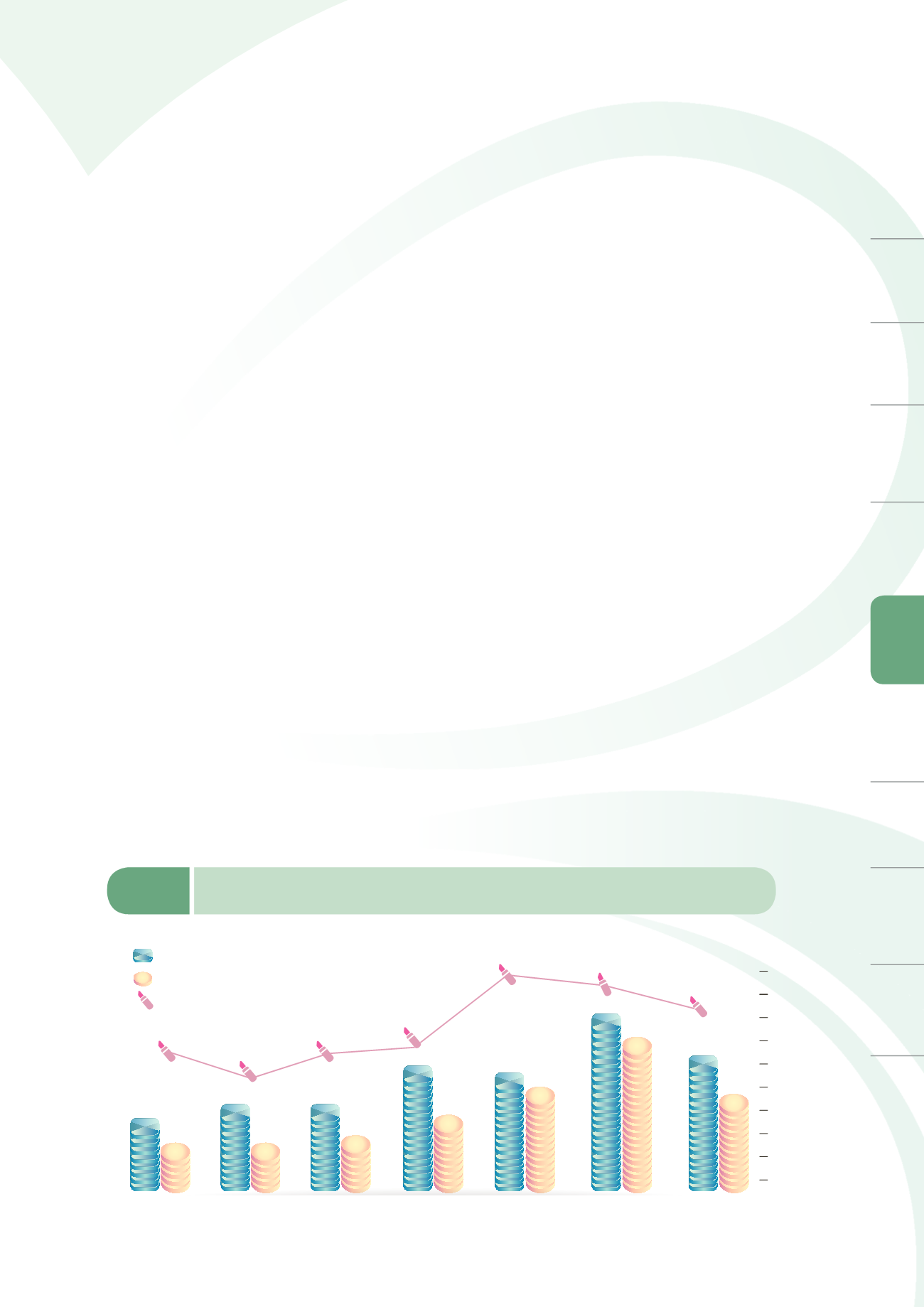
As of the end of 2014, a total of 103 cosmetics manufacturers have voluntarily applied for GMP
audits with IDB, of which 70 manufacturers have passed the audit. A total of 17 manufacturers
submitted their applications in 2014 with 12 passing the audits (approval rate of 70.6%) as shown in
Figure 6-4. Certified product categories include skin toners, skin balms, essences, shampoo, face
wash, shower foam, masks, lipsticks, chapsticks, lip gloss, pressed powder, and eye shadow.
55.6
0
40
50
30
10
20
60
70
80
90
9
5
0
10
5
15
20
25
30
2008
2009
2010
2011
2012
2013
2014
5
11
11
6
16
9
15
13
45.5
54.5
56.3
86.7
83.3
70.6
Number of manufacturers that have applied for GMP
Number of manufacturers qualified to GMP
Approval rate (%)
24
20
17
12
Figure 6-4
A list on the number of cosmetic manufacturers that have applied for and passed GMP
specifications from 2008 to 2014



















