
2015 Annual Report
51
Risk Assessment
Management and
Research Outcomes
Cosmetics
Management
Appendix
National
Laboratory and
Testing Network
Risk Communication
and Consumer
Protection
International
Cooperation and
Cross-Strait Exchange
Food
Management
Medicinal
Products
Management
Policy and
Organization
Controlled
Drugs
Management
Medical
Devices
Management
Section 4 Medical Device Safety Management
Current Status
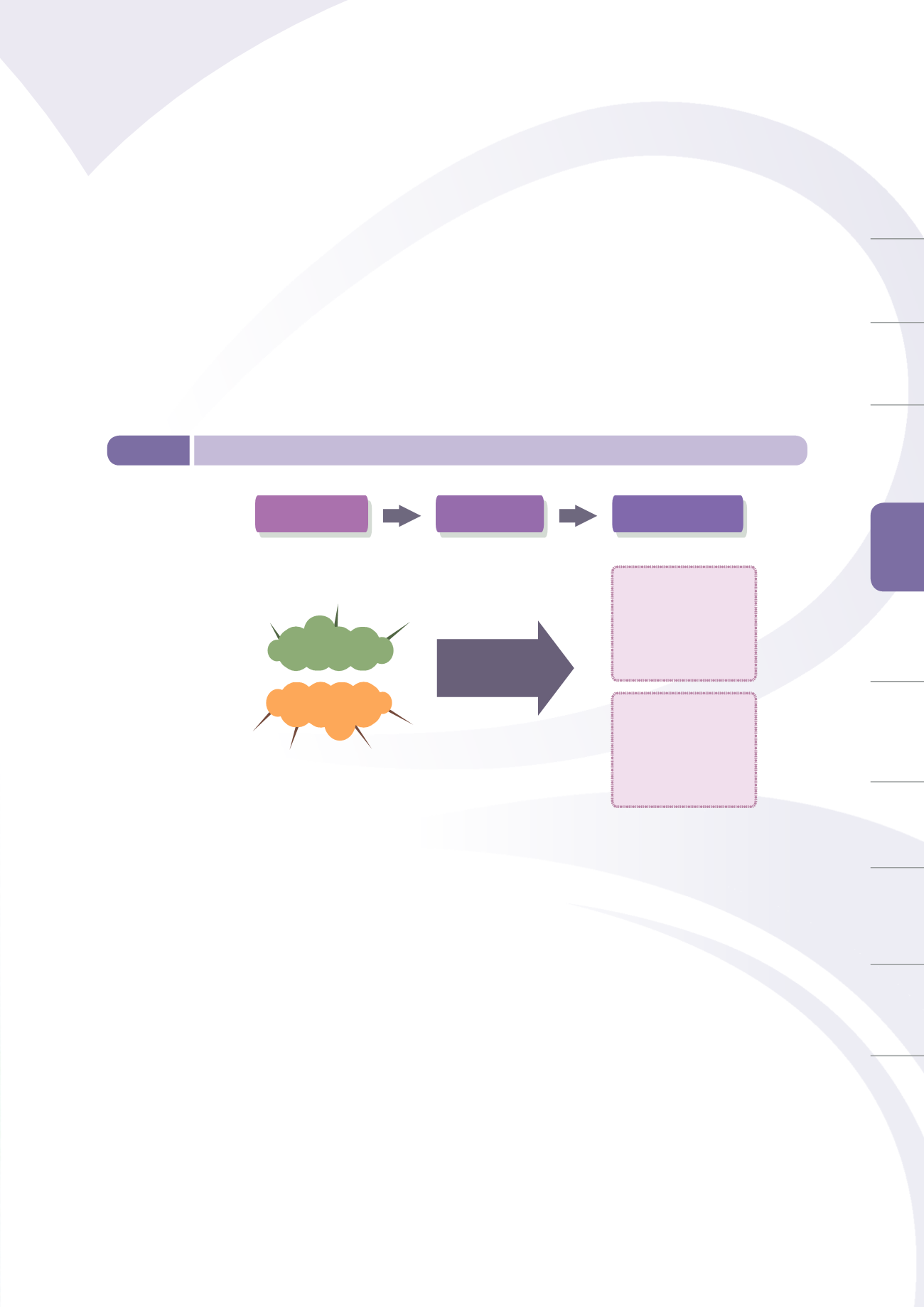
To establish a comprehensive safety surveillance mechanism and maintain post-market safety
surveillance of medical devices, TFDA has established an adverse device reaction (ADR) reporting
system, and been actively monitoring domestic and global medical device safety alerts, strengthening
distribution management of marketed goods, and promoting awareness of relevant regulations and
policies (Figure 5-4). The use of effective quality reporting systems allows for quick feedback, helps
regulatory agencies to exercise effective medical device safety management, and may propose
corrective and preventive measures to achieve a positive feedback cycle for excellent quality.
Policies and Outcomes
1. Strengthening Post-market Safety Monitoring of Medical Devices
(1) Active Monitoring of Domestic and Global Medical Device Safety Alerts
a. In 2014, a total of 1,454 safety alerts were received from the Safety Alert Dissemination System
(SADS) of the Asian Harmonization Working Party (AHWP).
b. In 2014, Taiwan's ADR Reporting System received a total of 1,401 cases of defective medical devices
and 209 cases of adverse device reactions.
c. In 2014, a total of 3,058 domestic and global safety alerts were actively monitored. These include
1,293 product advisories and 1,765 recall notifications. TFDA flagged 93 of these alerts as potentially
affecting the domestic public, and translated them into summaries for announcement.
(2) Joining the Global Vigilance Report Exchange System
TFDA is a member of the National Competent Authority exchange program (NCAR) of the
International Medical Device Regulators Forum (IMDRF) and receives recall notifications and safety
Surveillance
Safety
Quality
Analysis
Management
Active monitoring
of domestic and
global medical
device safety alerts
Adverse Device
Reaction (ADR)
Reporting System
Medical Device Safety
Monitoring (with Periodic
Safety Update Reports, PSUR)
Defective
Medical Device
Reporting
System Manufacturer
audit, joint audit
Active
monitoring of
domestic and
global medical
device quality
alerts
Postmarket
product quality
surveillance
program
Risk analysis
Re-evaluation
Risk controls
Amended package
inserts, restricted
use, extended
monitoring duration,
recalls, and product
withdrawals
Risk
communication
Education, training
and awareness work
and dissemination of
information
Figure 5-4
Post-market risk control mechanism of medical devices



















