
Food and Drug Administration
52
alerts issued by any member on a simultaneous basis. In 2014, a total of 331 global vigilance reports
were received. After evaluation, 93 of these were found to affect domestic market and related firms
were notified accordingly to take response measures.
(3) Improving ADR and Defective Product Reporting for Medical Devices
TFDA has, through promotion and awareness seminars, continued to encourage medical institutions,
manufacturers, and the general public to use the National ADR/Defective Product Reporting System
to report adverse events in order to enable the detection of post-market quality and safety issues of
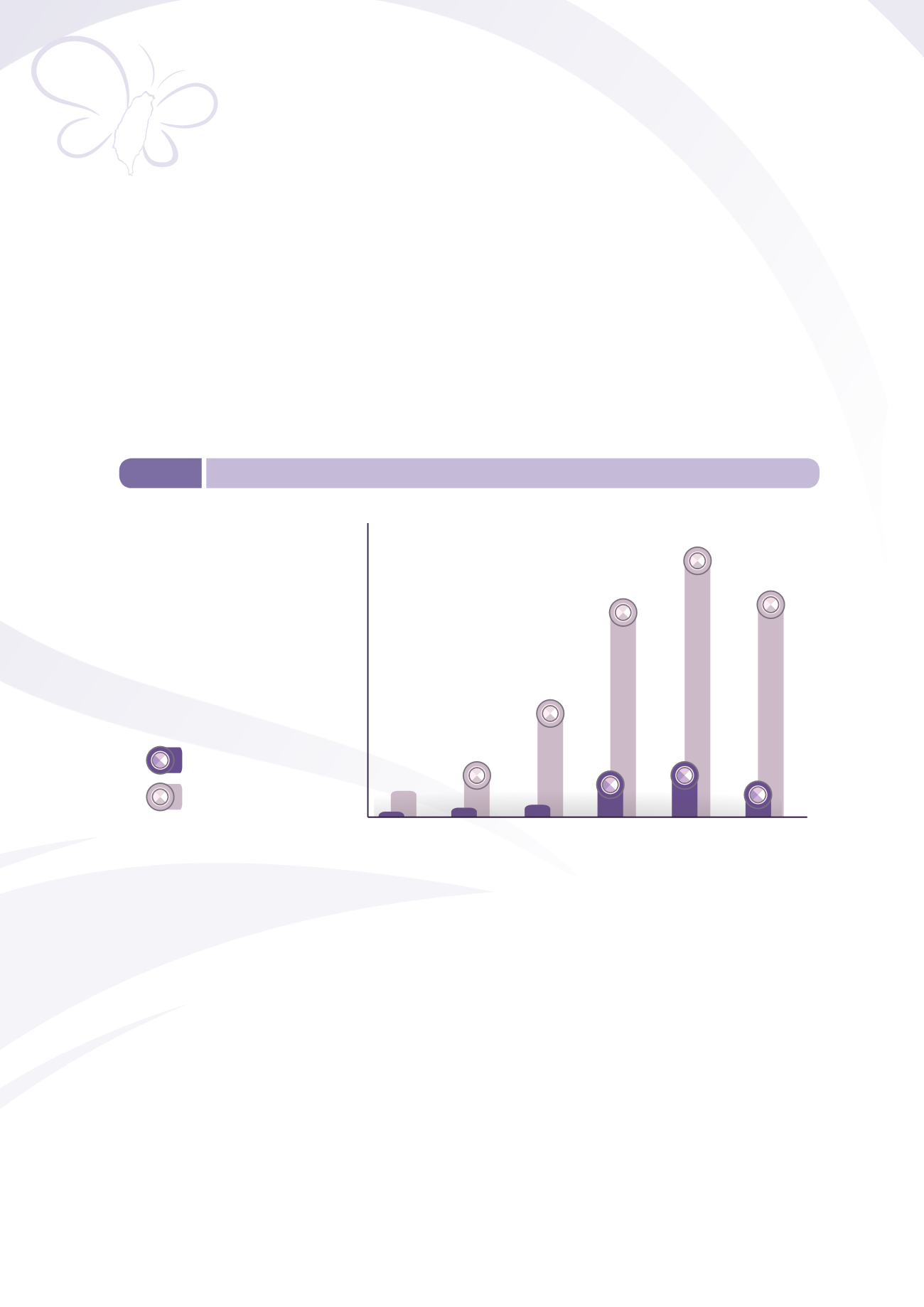
medical devices and the implementation of risk management measures. The number of reported ADR
rose from 30 cases in 2009 to 209 cases in 2014. Defective product reports rose from 157 to 1,401
cases over the same period (Figure 5-5).
2. Pharmaceutical Firm and Product Distribution Management
(1) Legalizing Online Sales for Class 1 and Some Class 2 Medical Devices
While ensuring public safety on the use of medical devices, TFDA has also been working to improve
convenience in acquiring such devices. According to its 1 November 2012 public notice
Registration
Requirements of Mail-Order Purchase for Medical Devices
, TFDA allows sales of Class 1 low-risk
medical devices and stipulates that dealers must have a pharmaceutical firm license permit for the
sales of medical devices as well as a physical business location and sales channel. Applications
must be submitted to the local health bureau in charge. Once approved, the medical device may
be marketed and sold through the Internet, television channels, and mail order. On 2 January 2014,
TFDA publicly announced three other Class 2 medical devices that can be sold through mail or online,
including condoms, tampons, and body fat meters for home use. Additionally, pharmaceutical firms
are required to specify on mail-order purchasing channels about certain information, such as reading
product instruction manuals carefully, regular calibration for products with measurement function, and
information about the manufacturing date and expiry date of the product.
2009
157
366
748
1368
1634
1401
2010
2011
2012
ADR Reports
Defective Product Reports
2013
2014
30
54
70
285
372
209
200
0
400
600
800
1000
1200
1400
1600
1800
Figure 5-5
Defective product / ADR reports of medical devices



















