
2015 Annual Report
49
Risk Assessment
Management and
Research Outcomes
Cosmetics
Management
Appendix
National
Laboratory and
Testing Network
Risk Communication
and Consumer
Protection
International
Cooperation and
Cross-Strait Exchange
Food
Management
Medicinal
Products
Management
Policy and
Organization
Controlled
Drugs
Management
Medical
Devices
Management
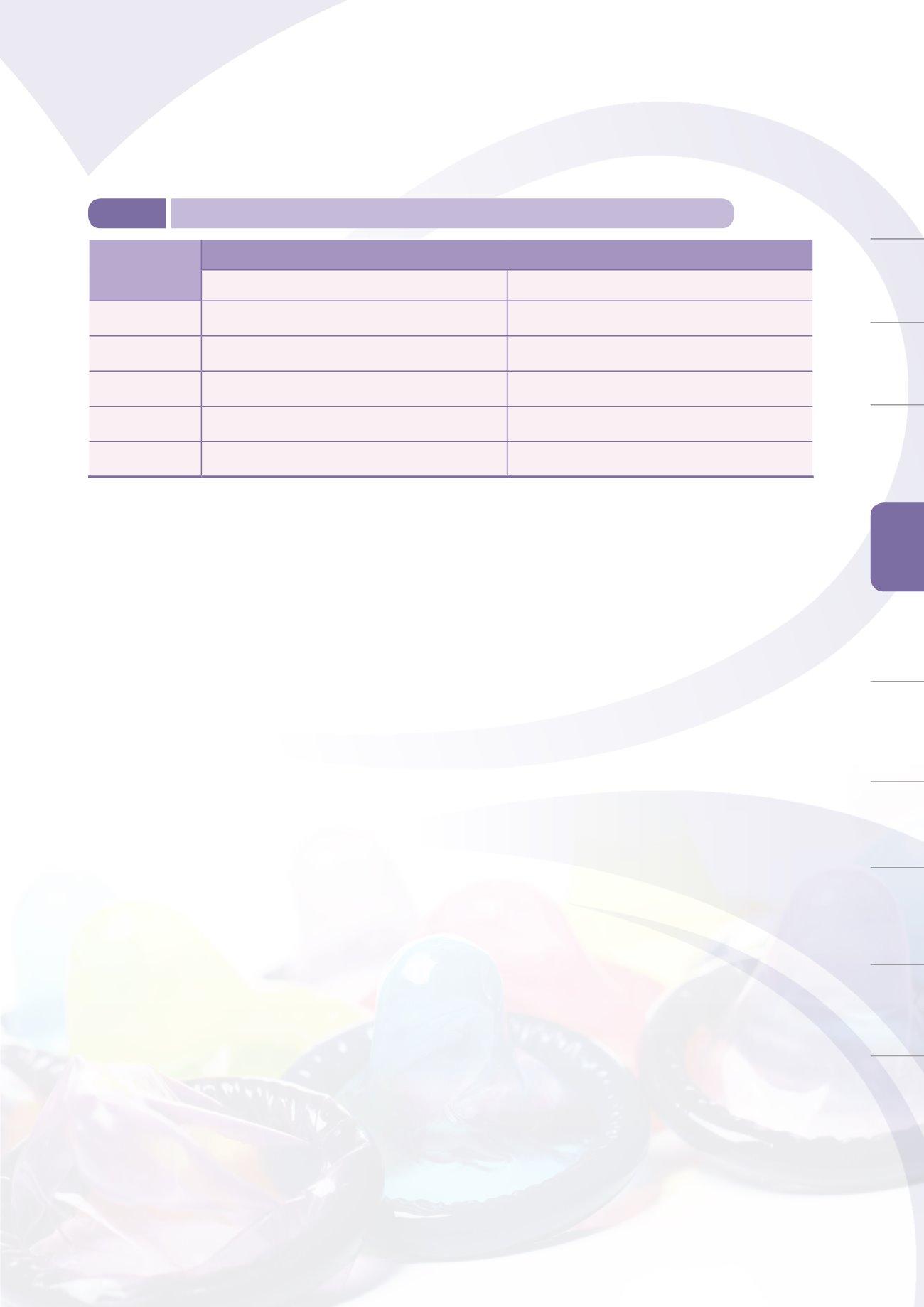
apply for on-site inspections of overseas manufacturing factories. By the end of 2014, there were
565 valid registration letters for domestic medical device GMP, and 3,057 registration letters for
imported medical device QSD (Table 5-1).
Section 3 Medical Device Quality Chain Monitoring
Current Status
Risk assessment mechanisms are used every year to target specified items. Resources from local
health bureaus are integrated in order to conduct the product quality monitoring plan. Post-market
quality surveillance is carried out by sampling marketed products offered in various drugstores,
pharmacies, medical device vendors, manufacturers or agents and subject them to quality
inspections.
Policies and Outcomes
1. Post-market Quality Surveillance of Medical Devices
Based on medical device management policy as well as items with higher nonconformities found
during post-market surveillance program, and adverse medical device reaction (ADR) reports, or
other risk assessment factors, TFDA carries out nationwide sampling inspections. In 2014, a total
of 216 items were sampled and subjected to quality testing as well as package labeling checks. Of
which, 205 items passed quality testing (qualified rate is 94.9%), and 129 items passed package
labeling checks (qualified rate is 59.7%). Nonconforming products were officially reported to the
local health bureau responsible for further administrative handling according to Pharmaceutical
Affairs Act. Results of various quality surveillance projects are shown in Table 5-2.
2. Joint Post-market Audit of Medical Devices
In order to effectively supervise the compliance of package labeling of marketed medical device
products, TFDA has provided support to local health bureaus by conducting joint medical device
audits. In 2014, a total of four medical device product categories were targeted for joint audits
due to their high rates of violation, risks, public scrutiny, and public sentiment. A total of 140 items
were audited, with 25 found to be nonconforming, a nonconforming rate of 17.9%. Major types of
violations included labeling and package inserts that violate the
Pharmaceutical Affairs Act.
Every
pharmaceutical company that committed package labeling violations for the medical devices has
been handled according to law by the local health bureau that exercise jurisdiction over them (Table
5-3).
Year
Number of vaild registration letters for medical device GMP / QSD
Valid GMP registration letters
Valid QSD registration letters
2010
236
1,340
2011
486
2,777
2012
531
3,065
2013
568
3,213
2014
565
3,057
Table 5-1
Number of valid registrations for medical device GMP / QSD



















