
2015 Annual Report
47
Risk Assessment
Management and
Research Outcomes
Cosmetics
Management
Appendix
National
Laboratory and
Testing Network
Risk Communication
and Consumer
Protection
International
Cooperation and
Cross-Strait Exchange
Food
Management
Medicinal
Products
Management
Policy and
Organization
Controlled
Drugs
Management
Medical
Devices
Management
comprehensive regulatory consultation network and strengthened the seamless three-step
counseling mechanism. TFDA is also actively identifying potential high-end innovative medical
device industries to provide with relevant consultation as well (Figure 5-3).
Consultation network
Three-Steps Consultation
Step 1:
Consulation on general
regulatory requirements
Step 3:
Project counseling
Step 2:
Consultation for
Individual Projects
Seed
instructors in the
industry,
academia,
and research fields
Center for
Drug Evaluation
TFDA,
Ministry of
Health and Welfare
Industry Communication
Platform (Regular)
Active Consultation
Online information and
Letter for consultation
Medical Device regulations
Counseling Center and
Inquiry Helpline
Figure 5-2
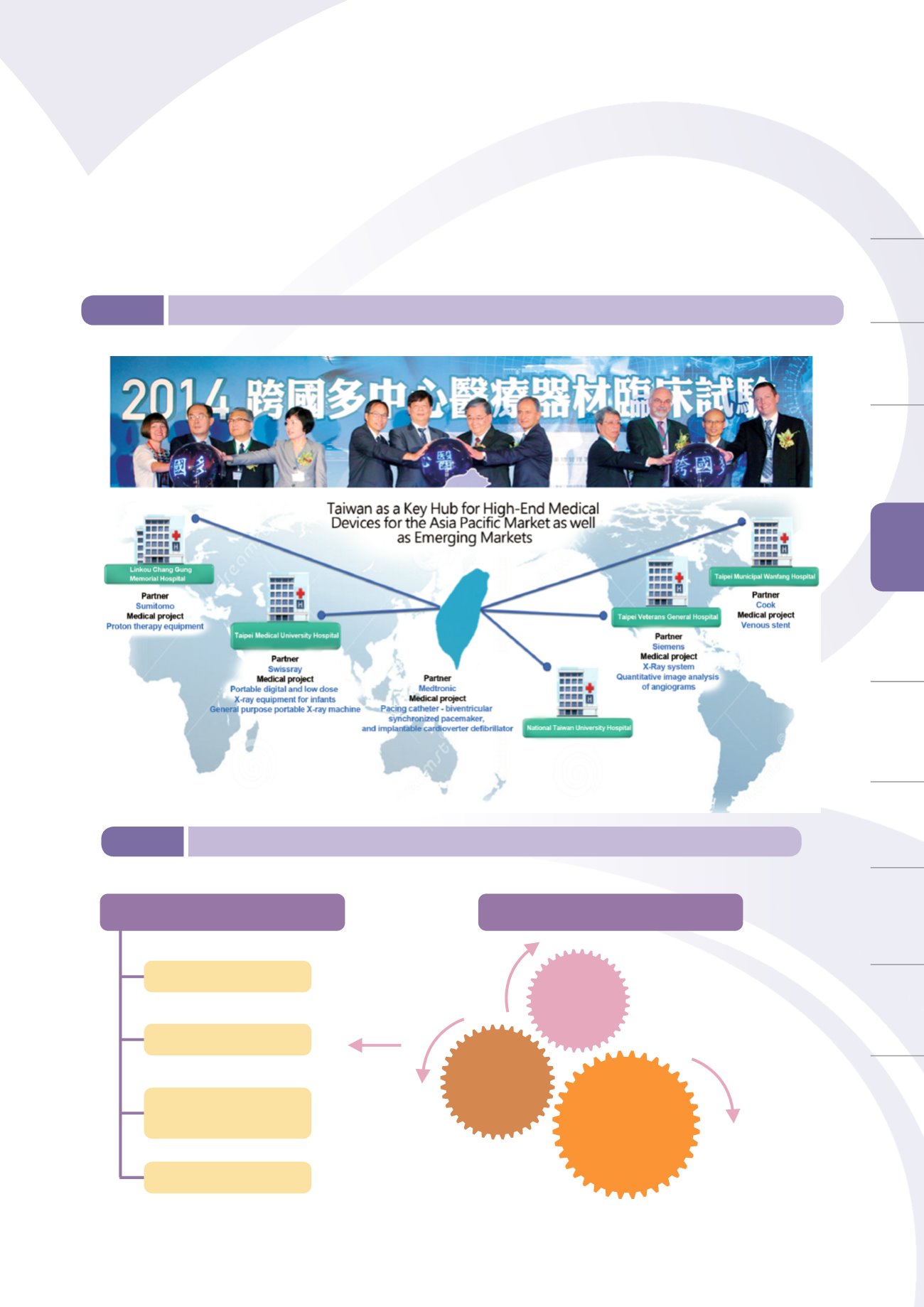
Taiwan's five major hospitals signing the multi-country medical device clinical trial partnership program
Figure 5-3
Comprehensive consultation program for medical device industry



















