
Food and Drug Administration
30
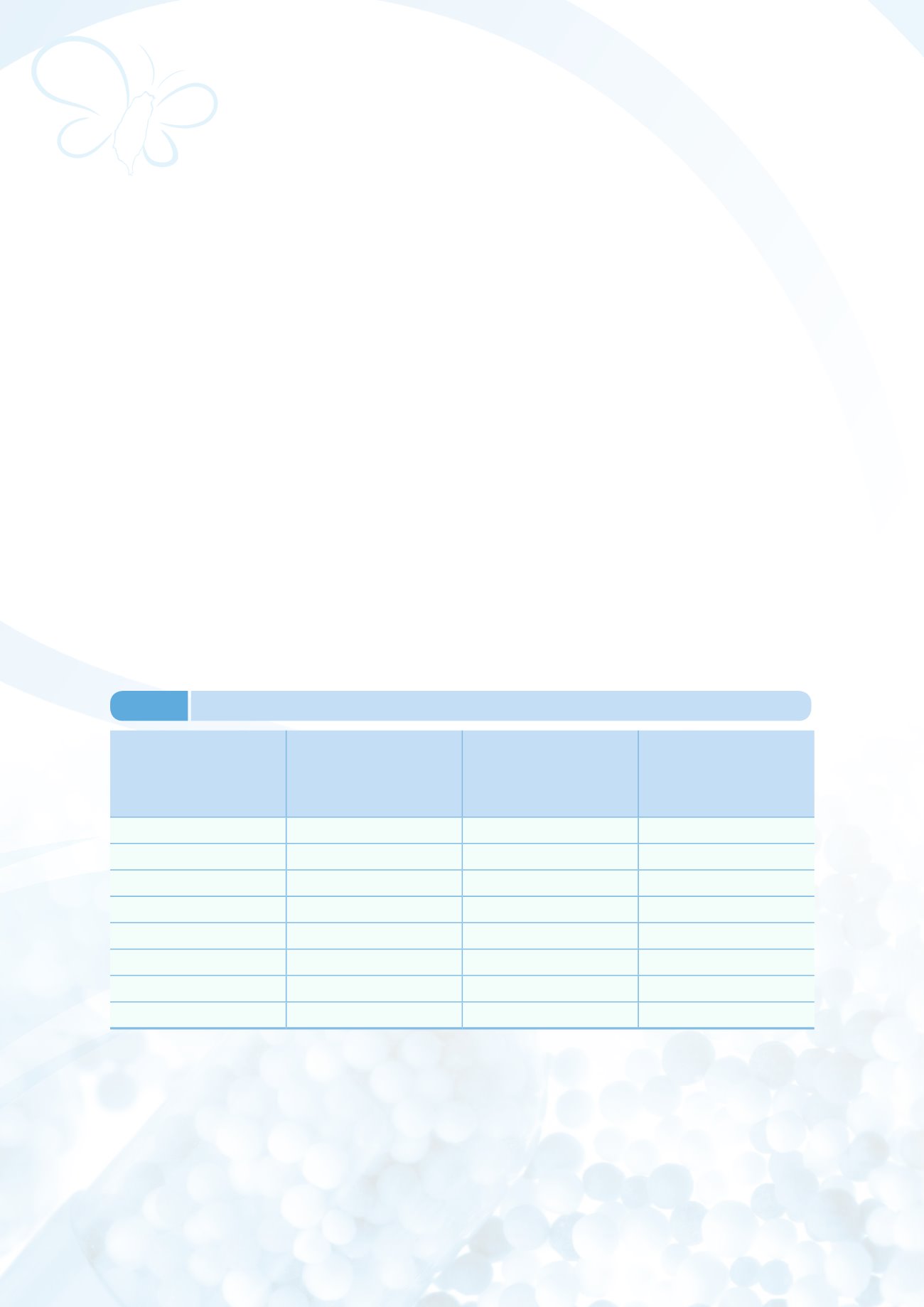
c. There were a total of 98 domestic GMP-compliant modern pharmaceutical manufacturers at the
end of December 2014, while 870 foreign manufacturers of imported pharmaceuticals have been
assessed as compliant. From 2002 to the end of December 2014, a total of 246 foreign modern
pharmaceutical manufacturers, coverd types of dosage forms, have passed on-site inspections,
ensuring a stable market supply of medicinal products. Implementation results are shown in Table
3-1.
d. To improve the quality of medicinal gases and prevent the risks of cross-contamination of different
gases, TFDA has provided consultation and promoted GMP and PIC/S GMP-compliance since
2002. Complete implementation of PIC/S GMP was achieved by 1 January 2014. By the end of
2014, a total of 34 medicinal gases manufacturers had complied with PIC/S GMP.
2. Source Management for Modern Pharmaceutical Manufacturers
a. The Drug Master File (DMF) system has been established to fortify the import management for
imported and self-use active pharmaceutical ingredients (API). From October 2009 to the end of
2014, a total of 2,089 DMF applications were handled and closed, of which 1,441 cases were
approved and 648 cases were rejected, representing an approval rate of 69%.
b. To strengthen the quality management of API manufacturers, the international PIC/S GMP Guide
was adopted as a compliance standard on 22 May 2013. In order to ensure API manufacturing
quality and improve global competitiveness of domestically manufactured APIs, a public
announcement was made on 25 September 2013 to achieve complete implementation of
API GMP by 31 December 2015. By the end of December 2014, a total of 21 domestic API
manufacturers and 163 items was found to be GMP-compliant.
Year
Number of GMP-
compliant domestic
modern pharmaceutical
manufacturers
Number of PIC/S GMP-
compliant domestic
modern pharmaceutical
manufacturers
Number of foreign
pharmaceutical
manufacturers found to be
GMP-compliant after on-site
inspection
2007
160
-
93
2008
151
-
118
2009
154
5
140
2010
155
22
157
2011
149
33
180
2012
145
44
209
2013
140
57
213
2014
98
98
246
Note: The numbers of domestic and foreign pharmaceutical manufacturers that have passed the assessments are
yearly accumulated figures.
Table 3-1
GMP-compliance of assessed domestic and foreign pharmaceutical manufacturers



















