
2015 Annual Report
27
Controlled
Drugs
Management
Medical
Devices
Management
Risk Assessment
Management and
Research Outcomes
Cosmetics
Management
Appendix
National
Laboratory and
Testing Network
Risk Communication
and Consumer
Protection
International
Cooperation and
Cross-Strait Exchange
Medicinal
Products
Management
Policy and
Organization
Food
Management
c. The conduction of clinical trials must follow the requirement
of Good Clinical Practice (GCP)
, which
asks the welfare and the rights of testing subjects and maintain the data quality and integrity. TFDA
also include the regular Contract Research Organization (CRO) inspections into GCT requirements
and 3 CROs were inspected during year 2014.
d.
In vivo
bioavailability/bioequivalence and
in vivo
dissolution studies are important methods for
demonstrating therapeutic equivalence between innovative and generic drugs. Until the end of
2014, there were 2,008 approved medicinal products had conducted BA/BE studies in Taiwan,
and 1,895 of them were domestic products.
(3) Innovation of New Drug Review and Approval Mechamism
New drug application (NDA) review procedures and time controls were promulgated on 22 May 2014
in order to improve drug review transparency, strengthen review process, and shorten overall review
time. TFDA has also referenced the review systems of Europe, the United States, Japan, and other
advanced countries and promulgated the
Abbreviated Review Procedure for New Drug Applications
,
the
Priority Review System for New Drug Applications
, and the
Pilot Program for Locally Developed
New Drug Applications
(Figure 3-3) in order to accelerate the market release of new drugs and satisfy
the treatment requirements of those with urgent medical needs. In 2014, a total of 135 NDAs were
approved, which included 29 local drugs and 106 imported drugs, with an average new drug review
length of 288 days which is better than some ICH memberships, like USA, Europen, Japan etc.
For example, Taigexyn Capsule, the locally developed new chemical entity (NCE) of nemonoxacin,
was the first approved by TFDA in the world. TFDA then provided consultation for post-market risk
management plans.
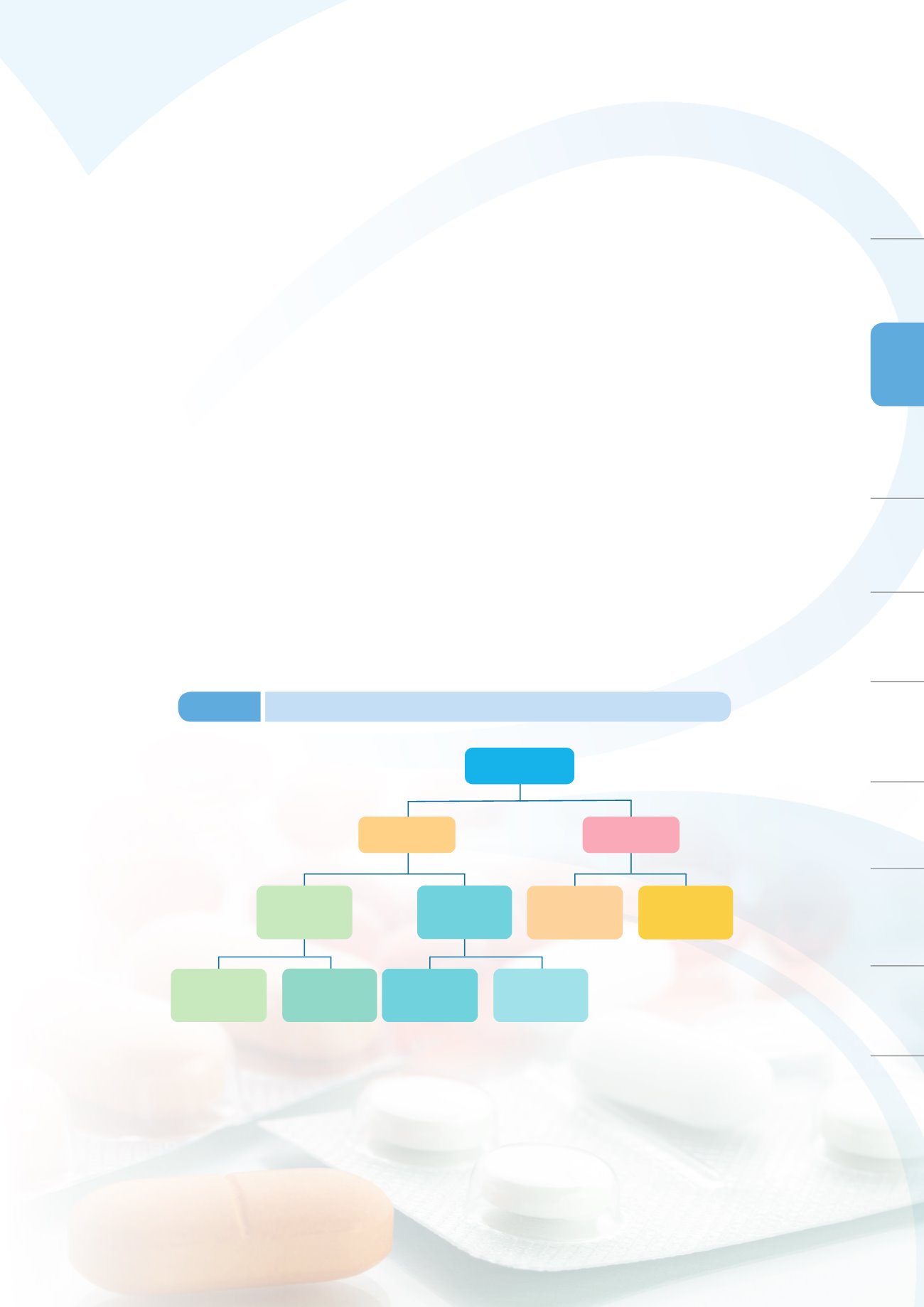
Priority review principles: NCE+ Serious Disease+ Unmet Medical Needs
Standard review
NCE
Regular Review
Regular review
Priority review
Priority review
Review Track for NDA
Abbreviated review
(new drugs withNCE)
Abbreviated review
(new drugs withNCE)
Standard review
Local innovative drugs
Special Pilot Project
Figure 3-3
Review track for NDA



















