
2015 Annual Report
29
Controlled
Drugs
Management
Medical
Devices
Management
Risk Assessment
Management and
Research Outcomes
Cosmetics
Management
Appendix
National
Laboratory and
Testing Network
Risk Communication
and Consumer
Protection
International
Cooperation and
Cross-Strait Exchange
Medicinal
Products
Management
Policy and
Organization
Food
Management
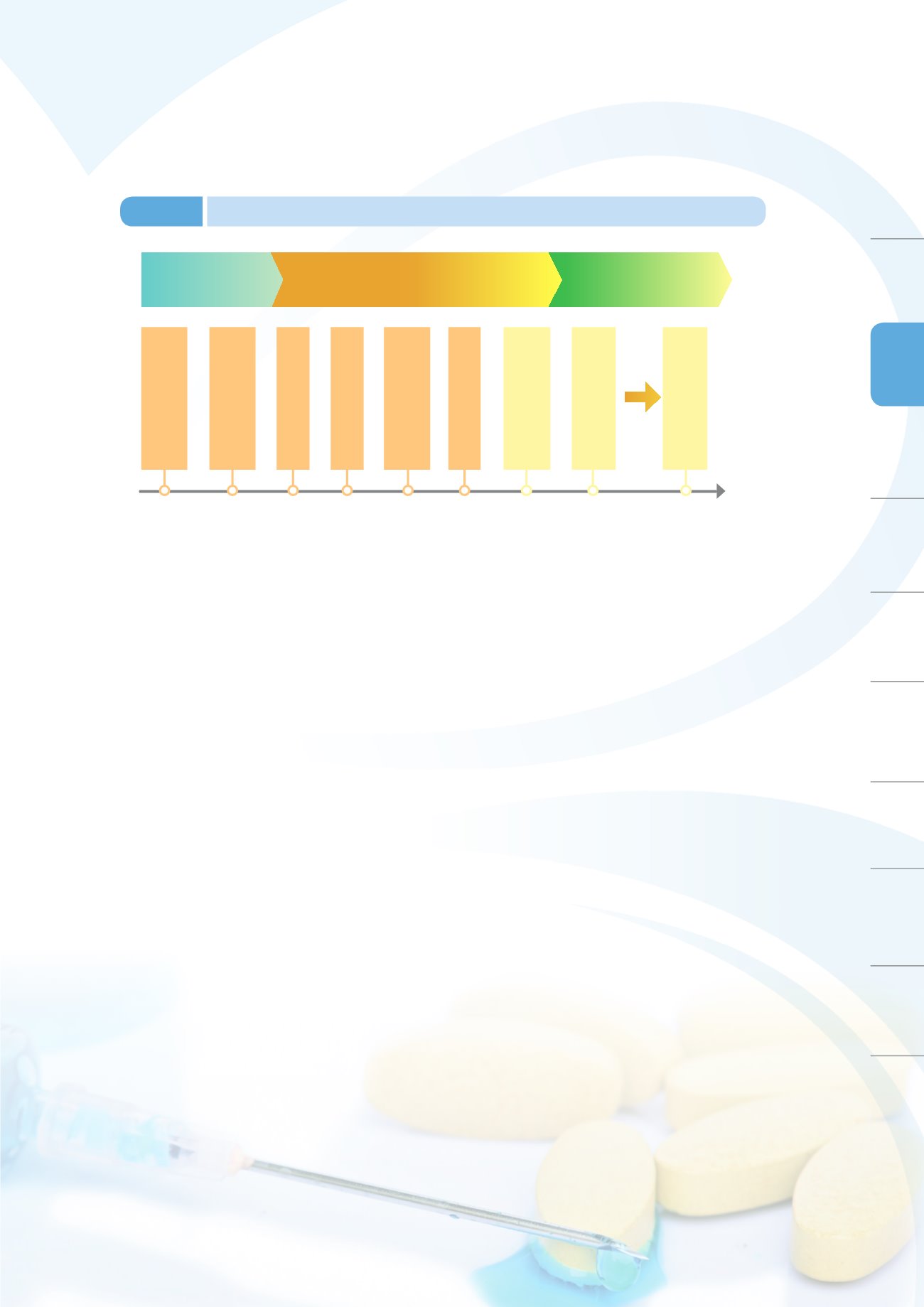
GMP
cGMP(current GMP)
Validations
PIC/S GMP
Japan visit and observations
by the Department of
Health and drafting of the
GMP outlines
Release of the
Pharmaceutical
GMP
1977
1982 1988 1995 1999 2005 December
2007
January
2010
Since 31 December
2014
Complete implementation
of the GMP
Complete implementation
of 3 validation stages
Validations of sterile
products
Public announcement of
implementing validation
to all
manufacturers/products
Public announcement of
PIC/S GMP implementation
Schedule
Promulgated PIC/S GMP
Complete implementation
of PIC/S GMP
An overview on the management scope of Taiwan's pharmaceutical GMP system shows that
the system started from modern pharmaceutical manufacturers and gradually incorporated
pharmaceutical distributors, medicinal gases manufacturers, and API manufacturers. In the
future, pharmaceutical Good Distribution Practice (GDP) will also be promoted and implemented
to ensure that quality management covers the entire pharmaceutical supply chain. To achieve
medicinal products source management and safeguard public safety in drug use, TFDA must
ensure conformance of PIC/S GMP amongst all modern pharmaceutical manufacturers, improve
pharmaceutical quality management of the manufacturers, and strengthen internal and external
supervision of the manufacturers.
Policies and Outcomes
1. Complete Compliance to the PIC/S GMP byModern Pharmaceutical Manufacturers
(1) Promotion of PIC/S GMP
a. In 1 January 2013, ahead of Japan and South Korea, Taiwan became an official member of
PIC/S, demonstrating international acceptance of the regulations, adminstration systems, and
inspection standards of pharmaceutical manufacturers in Taiwan. In 31 December 2014, TFDA
has successfully ensured that all modern pharmaceutical manufacturers have successfully
implemented the PIC/S GMP and fully comply with its requirements in order to continuously
safeguard drug use safety of the general public.
b. To continuously enforce stringent monitoring of medicinal products manufacturing, in addition
to conducting routine inspection of manufacturing sites every two-to-three years, unannounced
on-site inspections will be carried out in response to special incidents (such as whistle-blowing
events, post-market quality surveillance anomalies, and news events). For-cause inspection
plans must be used to verify whether the manufacturing sites are carrying out continuous
monitoring of medicinal product quality, by conducting on-site inspections and product sampling
as part of the quality monitoring measures to ensure that medicinal products quality within shelf
life are maintained.
Figure 3-5
History of implementing GMP amongst modern pharmaceutical manufacturers



















