
2015 Annual Report
35
Controlled
Drugs
Management
Medical
Devices
Management
Risk Assessment
Management and
Research Outcomes
Cosmetics
Management
Appendix
National
Laboratory and
Testing Network
Risk Communication
and Consumer
Protection
International
Cooperation and
Cross-Strait Exchange
Medicinal
Products
Management
Policy and
Organization
Food
Management
Identifying the
Problem
Analyzing the
Problem
Solving the
Problem
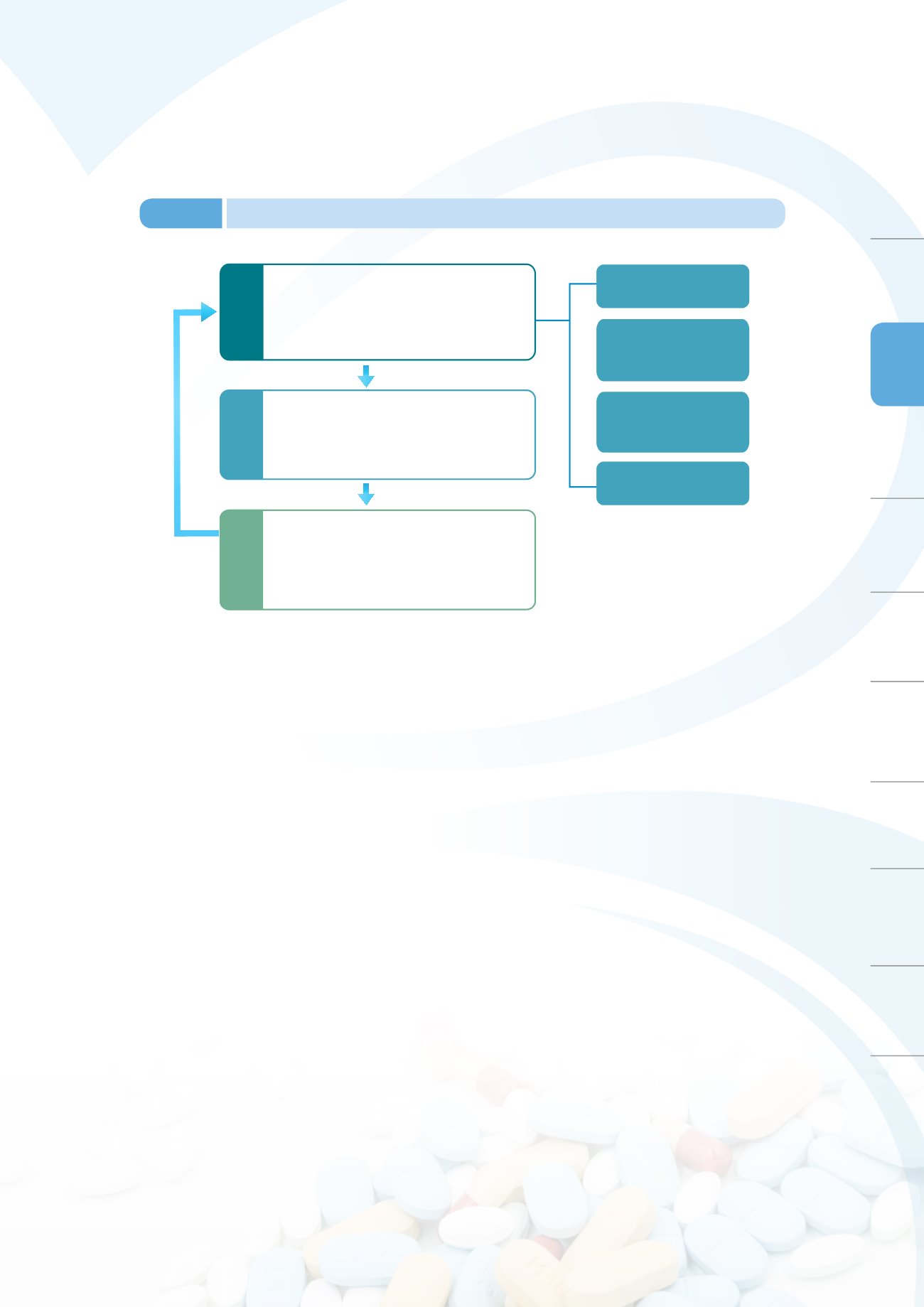
Safety monitoring
Identifying potential safety problems
arising from drug use through the
spontaneous reporting system most
commonly used currently
Adverse reactions
reporting system
Regular safety reports
during medicinal product
surveillance periods
Surveillance of domestic
and global alerts
Active surveillance
systems for medicinal
product safety
Safety analysis and evaluations
Scientific methods are employed to identify
potential safety issues in the medicinal
product and evaluate its clinical benefits and
risks (signal refinement)
Risk Control
In response to medicinal product safety issues,
appropriate risk control measures must be taken
(including revision of package inserts, usage
restrictions, and withdrawing the product
from the market)
(2) New Drug Safety Monitoring
Regulations Governing Safety Monitoring of Medicines
was announced in 2004, and
safety
monitoring period
is required for new drugs. During the period of drug safety monitoring, license
holders shall collect safety information on drug used both domestically and abroad and provide
periodic safety update reports. From 2004 to the end of 2014, a total of 291 new medicines are
under new drug safety monitoring.
(3) Monitoring Domestic and Global Drug Safety Alerts
The ADR center and TFDA monitor domestic and international drug safety alerts, for issue warnings
to healthcare professional and the public. A total of 167 drug safety alerts were monitored in 2014.
(4) Proactive Drug Safety Monitoring Mechanism
The proactive assessment of drug safety mechanism was established since 2010. Such mechanisms
actively analyze the post-market drug safety of high-risk drugs by using national health insurance data
as reference for drug safety re-evaluation or planning of risk management.
2. Re-evaluation of Drug Safety and Risk Management
For drugs with safety concerns, domestic and global data were collected to reevaluate drug
safety. In 2014, there were 59 drugs re-evaluated, among them, 22 were required for risk
management measures, including 21 safety labeling changes or restricted use, Chloramphenicol
and ketoconazole oral dosage products were asked to be off the marketd due to its risk.
Figure 3-8
Procedure for post-market safety controls for medicinal products



















