
Food and Drug Administration
26
Policies and Outcomes
1. Comprehensive Regulation
0TWVY[HU[ YL]PZPVUZ [V TLKPJPUHS WYVK\J[ THUHNLTLU[ YLN\SH[PVUZ HUK Z[HUKHYKZ PU
PUJS\KL
advertisements for orally administered western medicine containing alcohol, regulations for registration
VM TLKPJPUHS WYVK\J[Z UL^ KY\N HWWSPJH[PVUZ 5+( ZHML[` Z[HUKHYKZ MVY UVU JSPUPJHS Z[\K` VM TLKPJPUHS
products, clinical trial applications for human cellular therapy products, advertisements for solutions
JVU[HPUPUN JVKLPUL VY JHMMLPUL .VVK *SPUPJHS 7YHJ[PJL MVY TLKPJPUHS WYVK\J[Z JVTWHYHIPSP[` L_LYJPZL
Z[HUKHYKZ MVY IPV[LJOUVSVN` IPVSVNPJHS KY\NZ HUK SPZ[ VM HJJLW[LK ZWLJPÄJH[PVUZ MYVT [OL 0U[LYUH[PVUHS
*VUMLYLUJL VM /HYTVUPaH[PVU 0*/ -VY KL[HPSZ WSLHZL YLMLY [V (WWLUKP_ (UUL_ ;HISL
2. Registration of Medicinal Products
4LKPJPUHS WYVK\J[ YLNPZ[YH[PVU JHU IL KP]PKLK PU[V HJ[P]L WOHYTHJL\[PJHS PUNYLKPLU[ (70 HUK P[Z
preparations. Preparations can be further divided into new drugs, biological products, generic drugs
HUK VYWOHU KY\NZ >OLYL SVJHS JSPUPJHS [YPHSZ VY IPVH]HPSHIPSP[` )( HUK IPVLX\P]HSLUJL ), Z[\KPLZ HYL
YLX\PYLK HZ H[[HJOTLU[Z MVY PUZWLJ[PVU HUK YLNPZ[YH[PVU HWWSPJH[PVUZ [OL JVYYLZWVUKPUN WYVQLJ[ WSHUZ
and reports must be reviewed as well.
4LKPJPUHS 7YVK\J[ 0UZWLJ[PVU HUK 9LNPZ[YH[PVU ILMVYL 4HYRL[ 9LSLHZL
H ;-+( MVSSV^Z [OL NSVIHS Z[HUKHYK [OH[ Z[YLZZLZ VU [OL PTWVY[HUJL VM [OL :HML[` ,MÄJHJ` HUK 8\HSP[`
HUK ;-+( HSZV PTWSLTLU[Z [OL *VTTVU ;LJOUPJHS +VJ\TLU[ *;+ MVY TLKPJPUHS WYVK\J[ HWWYV]HS
system.
I (Z VM
;-+( OHK PZZ\LK H [V[HS VM
WOHYTHJL\[PJHS SPJLUZLZ ^OPJO PUJS\KLK
HJ[P]L WOHYTHJL\[PJHS PUNYLKPLU[Z HUK WOHYTHJL\[PJHS ÄUPZOLK WYVK\J[Z NLULYPJ KY\NZ
UL^ KY\NZ IPVSVNPJZ HUK VYWOHU KY\NZ -PN\YL
:[H[PZ[PJHS KH[H VU HWWYV]LK KY\N SPJLUZLZ PZZ\LK
L]LY` `LHY HYL WYV]PKLK PU (WWLUKP_ (UUL_ ;HISL
*SPUPJHS ;YPHS 9L]PL^Z
H )` YL]PZPUN [OL
.\PKHUJL MVY 0U]LZ[PNH[PVUHS 5L^ +Y\N (WWSPJH[PVUZ
and the establishment of
*LU[YHSPaLK *SPUPJHS ;YHPS ,[OPJZ 9L]PL^ JLU[YHS 09)
TLJOHUPZT ;-+( KLKPJH[LZ PU HJJLSLYH[PUN [OL
05+ YL]PL^ WYVJLZZ
I 0U
;-+( YLJLP]LK H [V[HS VM
UL^ 05+ HWWSPJH[PVUZ HUK
05+ HTLUKTLU[Z ;OL
growth rate of submission is 1.2 times more than 2013.
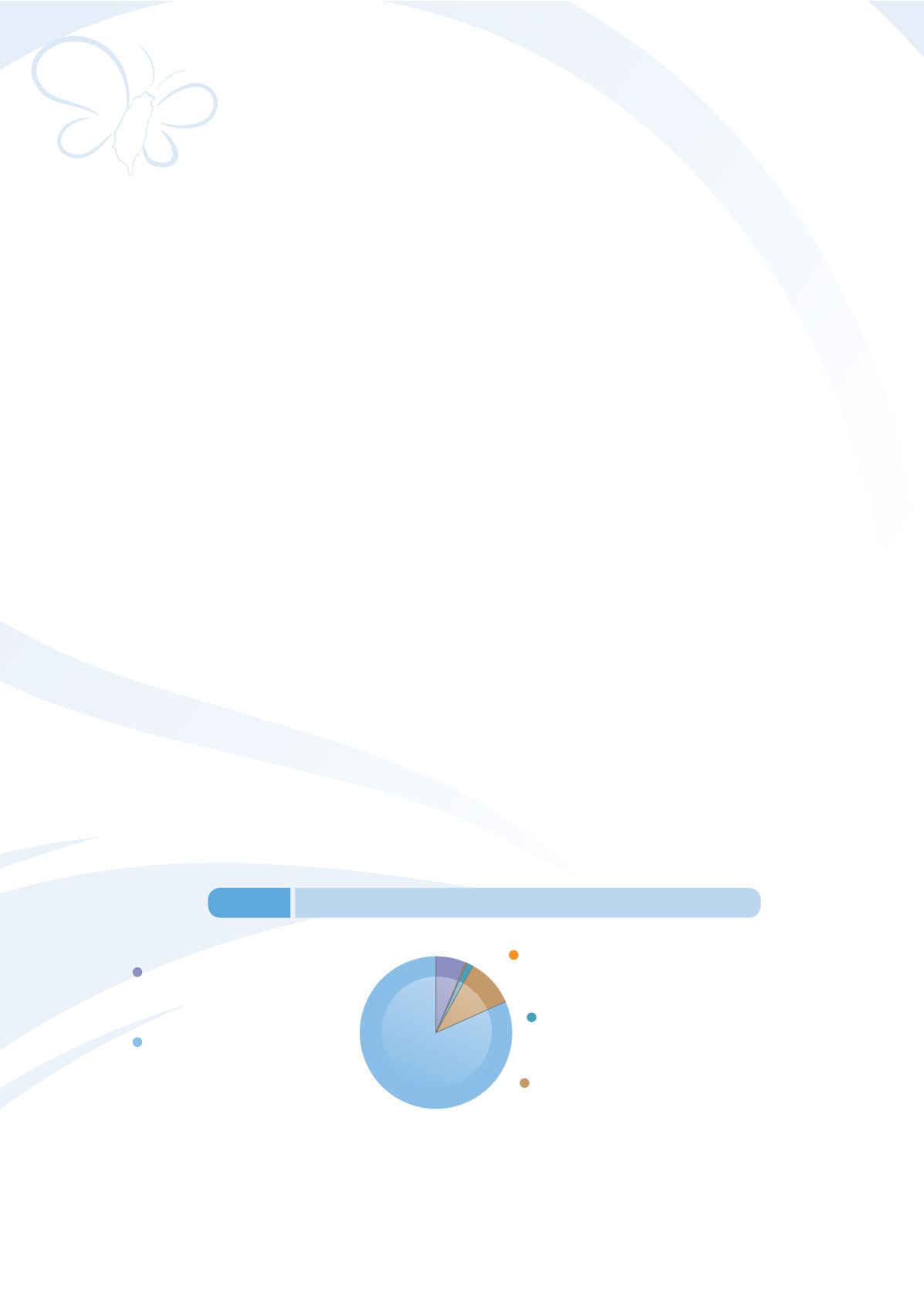
Genetic drugs 21,785
licenses, 81.64%
(19,531 domestic drug licenses;
2,254 imported drug licenses)
New drugs 1,771 licenses, 6.64%
(495 domestic drug licenses;
1,276 imported drug licenses)
(10 domestic drug licenses;
41 imported drug licenses)
Orphan drugs 51 licenses, 0.19%
(22 domestic drug licenses;
337 imported drug licenses)
Biological products 359 licenses, 1.35%
(602 domestic drug licenses;
2,115 imported drug licenses)
APIs 2,717 licenses, 10.18%
Figure 3-2
Statistics on pharmaceutical (2014)



















