Page 102 - 2020Taiwan Food and Drug Administration Annual Report
P. 102
%BUF PG
/BNF *NQPSUBOU DPOUFOU
BOOPVODFNFOU
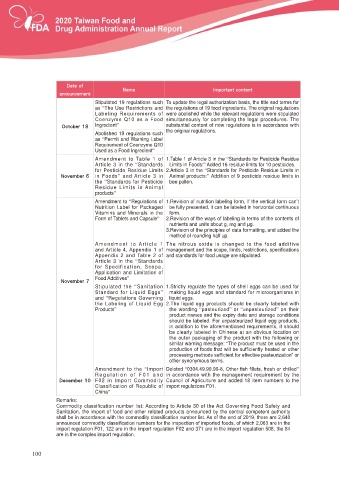
Stipulated 19 regulations such 7R XSGDWH WKH OHJDO DXWKRUL]DWLRQ EDVLV WKH WLWOH DQG WHUPV IRU
as “The Use Restrictions and the regulations of 19 food ingredients. The original regulations
/DEHOLQJ 5HTXLUHPHQWV RI were abolished while the relevant regulations were stipulated
&RHQ]\PH 4 DV D )RRG simultaneously for completing the legal procedures. The
0DUPCFS Ingredient” substantial content of new regulations is in accordance with
Abolished 19 regulations such the original regulations.
as “Permit and Warning Label
5HTXLUHPHQW RI &RHQ]\PH 4
Used as a Food Ingredient”
Amendment to Table 1 of 1.Table 1 of Article 3 in the “Standards for Pesticide Residue
Article 3 in the “Standards Limits in Foods:” Added 16 residue limits for 10 pesticides.
for Pesticide Residue Limits 2.Article 3 in the “Standards for Pesticide Residue Limits in
/PWFNCFS in Foods” and Article 3 in Animal products:” Addition of 9 pesticide residue limits in
the “Standards for Pesticide bee pollen.
Residue Limits in Animal
products”
Amendment to “Regulations of 1.Revision of nutrition labeling form, If the vertical form can’t
Nutrition Label for Packaged EH IXOO\ SUHVHQWHG LW FDQ EH ODEHOHG LQ KRUL]RQWDO FRQWLQXRXV
Vitamins and Minerals in the form.
Form of Tablets and Capsule” 2.Revision of the ways of labeling in terms of the contents of
QXWULHQWV DQG XQLWV DERXW J PJ DQG ȝJ
3.Revision of the principles of data formatting, and added the
method of rounding half up.
Amendment to Article 1 7KH QLWURXV R[LGH LV FKDQJHG WR WKH IRRG DGGLWLYH
DQG $UWLFOH $SSHQGL[ RI PDQDJHPHQW DQG WKH VFRSH OLPLWV UHVWULFWLRQV VSHFL¿FDWLRQV
$SSHQGL[ DQG 7DEOH RI and standards for food usage are stipulated.
Article 3 in the “Standards
for Specification, Scope,
Application and Limitation of
Food Additives”
/PWFNCFS
Stipulated the “Sanitation 1.Strictly regulate the types of shell eggs can be used for
6WDQGDUG IRU /LTXLG (JJV” PDNLQJ OLTXLG HJJV DQG VWDQGDUG IRU PLFURRUJDQLVPV LQ
and “Regulations Governing OLTXLG HJJV
WKH /DEHOLQJ RI /LTXLG (JJ 7KH OLTXLG HJJ SURGXFWV VKRXOG EH FOHDUO\ ODEHOHG ZLWK
Products” the wording “SDVWHXUL]HG” or “XQSDVWHXUL]HG” on their
SURGXFW QDPHV DQG WKH H[SLU\ GDWH DQG VWRUDJH FRQGLWLRQV
VKRXOG EH ODEHOHG )RU XQSDVWHXUL]HG OLTXLG HJJ SURGXFWV
LQ DGGLWLRQ WR WKH DIRUHPHQWLRQHG UHTXLUHPHQWV LW VKRXOG
be clearly labeled in Chinese at an obvious location on
the outer packaging of the product with the following or
similar warning message: “The product must be used in the
SURGXFWLRQ RI IRRGV WKDW ZLOO EH VXI¿FLHQWO\ KHDWHG RU RWKHU
SURFHVVLQJ PHWKRGV VXI¿FLHQW IRU HIIHFWLYH SDVWHXUL]DWLRQ´ RU
other synonymous terms.
Amendment to the “Import Deleted “ 2WKHU ¿VK ¿OOHWV IUHVK RU FKLOOHG”
Regulation of F01 and LQ DFFRUGDQFH ZLWK WKH PDQDJHPHQW UHTXLUHPHQW E\ WKH
%FDFNCFS F02 in Import Commodity Council of Agriculture and added 18 item numbers to the
Classification of Republic of import regulations F01.
China”
Remarks:
Commodity classification number list: According to Article 30 of the Act Governing Food Safety and
Sanitation, the import of food and other related products announced by the central competent authority
VKDOO EH LQ DFFRUGDQFH ZLWK WKH FRPPRGLW\ FODVVL¿FDWLRQ QXPEHU OLVW $V RI WKH HQG RI WKHUH DUH
DQQRXQFHG FRPPRGLW\ FODVVL¿FDWLRQ QXPEHUV IRU WKH LQVSHFWLRQ RI LPSRUWHG IRRGV RI ZKLFK DUH LQ WKH
import regulation F01, 122 are in the import regulation F02 and 371 are in the import regulation 508, the 84
DUH LQ WKH FRPSOH[ LPSRUW UHJXODWLRQ
100