Page 99 - 2020Taiwan Food and Drug Administration Annual Report
P. 99
%BUF PG
/BNF *NQPSUBOU DPOUFOU
BOOPVODFNFOU
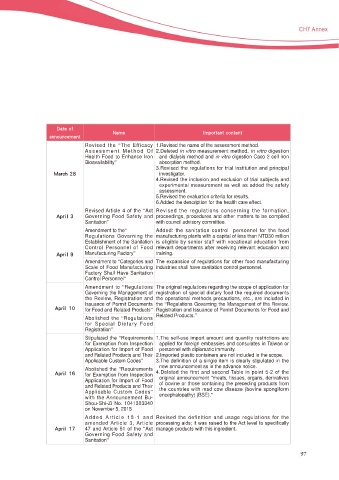
Revised the “The Efficacy 1.Revised the name of the assessment method.
Assessment Method Of 2.Deleted in vitro measurement method, in vitro digestion
Health Food to Enhance Iron and dialysis method and in vitro digestion Caco 2 cell iron
Bioavailability” absorption method.
3.Revised the regulations for trial institution and principal
.BSDI investigator.
5HYLVHG WKH LQFOXVLRQ DQG H[FOXVLRQ RI WULDO VXEMHFWV DQG
H[SHULPHQWDO PHDVXUHPHQW DV ZHOO DV DGGHG WKH VDIHW\
assessment.
5.Revised the evaluation criteria for results.
6.Added the description for the health care effect.
Revised Article 4 of the “Act Revised the regulations concerning the formation,
"QSJM Governing Food Safety and proceedings, procedures and other matters to be complied
Sanitation” with council advisory committee.
Amendment to the“ Added: the sanitation control personnel for the food
Regulations Governing the manufacturing plants with a capital of less than NTD30 million
Establishment of the Sanitation is eligible by senior staff with vocational education from
Control Personnel of Food relevant departments after receiving relevant education and
"QSJM Manufacturing Factory” training.
Amendment to “Categories and 7KH H[SDQVLRQ RI UHJXODWLRQV IRU RWKHU IRRG PDQXIDFWXULQJ
Scale of Food Manufacturing industries shall have sanitation control personnel.
Factory Shall Have Sanitation
Control Personnel”
Amendment to “Regulations The original regulations regarding the scope of application for
Governing the Management of UHJLVWUDWLRQ RI VSHFLDO GLHWDU\ IRRG WKH UHTXLUHG GRFXPHQWV
the Review, Registration and the operational methods precautions, etc., are included in
Issuance of Permit Documents the “Regulations Governing the Management of the Review,
"QSJM for Food and Related Products” Registration and Issuance of Permit Documents for Food and
Related Products.”
Abolished the “Regulations
for Special Dietary Food
Registration”
Stipulated the “5HTXLUHPHQWV 7KH VHOI XVH LPSRUW DPRXQW DQG TXDQWLW\ UHVWULFWLRQV DUH
IRU ([HPSWLRQ IURP ,QVSHFWLRQ applied for foreign embassies and consulates in Taiwan or
Application for Import of Food personnel with diplomatic immunity.
and Related Products and Their 2.Imported plastic containers are not included in the scope.
Applicable Custom Codes” 3.The definition of a single item is clearly stipulated in the
Abolished the “5HTXLUHPHQWV new announcement as in the advance notice.
"QSJM IRU ([HPSWLRQ IURP ,QVSHFWLRQ 4.Deleted the first and second Table in point 5-2 of the
Application for Import of Food original announcement “meats, tissues, organs, derivatives
of bovine or those containing the preceding products from
and Related Products and Their the countries with mad cow disease (bovine spongiform
Applicable Custom Codes”
with the Announcement Bu- encephalopathy) (BSE).”
Shou-Shi-Zi No. 1041303340
on November 5, 2015
Added Article 18-1 and Revised the definition and usage regulations for the
amended Article 3, Article SURFHVVLQJ DLGV LW ZDV UDLVHG WR WKH $FW OHYHO WR VSHFL¿FDOO\
"QSJM 47 and Article 51 of the “Act manage products with this ingredient.
Governing Food Safety and
Sanitation”
97