Page 125 - 2018食藥署年報(英文版)
P. 125
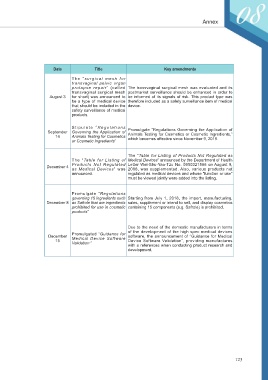
Date Title Key amendments
The “surgical mesh for
transvaginal pelvic organ
prolapse repair” (called The transvaginal surgical mesh was evaluated and its
transvaginal surgical mesh postmarket surveillance should be enhanced in order to
August 3 for short) was announced to be informed of its signals of risk. This product type was
be a type of medical device therefore included as a safety surveillance item of medical
that should be included in the device.
safety surveillance of medical
products.
St ipulat e “ Regulat ions
September Governing the Application of Promulgate “Regulations Governing the Application of
Animals Testing for Cosmetics or Cosmetic Ingredients,”
14 Animals Testing for Cosmetics which becomes effective since November 9, 2019.
or Cosmetic Ingredients”
The “Table for Listing of Products Not Regulated as
The “Table for Listing of Medical Devices” announced by the Department of Health
Products Not Regulated Letter Wei-Shu-Yao-Tzu No. 0950321586 on August 9,
December 4
as Medical Devices” was 2006, was supplemented. Also, various products not
announced. regulated as medical devices and whose “function or use”
must be viewed jointly were added into the listing.
Promulgate “Regulations
governing 15 ingredients such Starting from July 1, 2018, the import, manufacturing,
December 8 as Safrole that are ingredients sales, supplement or intend to sell, and display cosmetics
prohibited for use in cosmetic containing 15 components (e.g. Safrole) is prohibited.
products”
Due to the need of the domestic manufacturers in terms
of the development of the high spec medical devices
Promulgated “Guidance for
December Medical Device Software software, the announcement of “Guidance for Medical
15 Device Software Validation”, providing manufactures
Validation ”
with a references when conducting product research and
development.
123