Page 25 - 2017食品藥物管理署年報(英文版)
P. 25
2017 Taiwan Food and Drug Administration Annual Report Chapter 2. Legal Environment and Registration
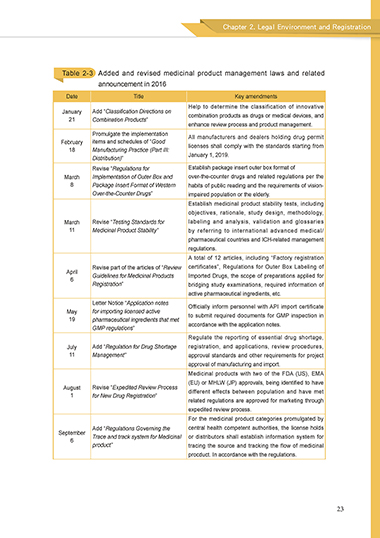
Table 2-3 Added and revised medicinal product management laws and related
announcement in 2016
Date Title Key amendments
Help to determine the classification of innovative
January Add “Classification Directions on combination products as drugs or medical devices, and
21 Combination Products”
enhance review process and product management.
Promulgate the implementation All manufacturers and dealers holding drug permit
February items and schedules of “Good licenses shall comply with the standards starting from
18 Manufacturing Practice (Part III:
Distribution)” January 1, 2019.
Revise “Regulations for Establish package insert outer box format of
March Implementation of Outer Box and over-the-counter drugs and related regulations per the
8 Package Insert Format of Western habits of public reading and the requirements of vision-
Over-the-Counter Drugs” impaired population or the elderly.
Establish medicinal product stability tests, including
objectives, rationale, study design, methodology,
March Revise “Testing Standards for labeling and analysis, validation and glossaries
11 Medicinal Product Stability” by referring to international advanced medical/
pharmaceutical countries and ICH-related management
regulations.
A total of 12 articles, including “Factory registration
Revise part of the articles of “Review certificates”, Regulations for Outer Box Labeling of
April Guidelines for Medicinal Products Imported Drugs, the scope of preparations applied for
6
Registration” bridging study examinations, required information of
active pharmaceutical ingredients, etc.
Letter Notice “Application notes Officially inform personnel with API import certificate
May for importing licensed active to submit required documents for GMP inspection in
19 pharmaceutical ingredients that met
GMP regulations” accordance with the application notes.
Regulate the reporting of essential drug shortage,
July Add “Regulation for Drug Shortage registration, and applications, review procedures,
11 Management” approval standards and other requirements for project
approval of manufacturing and import.
Medicinal products with two of the FDA (US), EMA
(EU) or MHLW (JP) approvals, being identified to have
August Revise “Expedited Review Process different effects between population and have met
1 for New Drug Registration”
related regulations are approved for marketing through
expedited review process.
For the medicinal product categories promulgated by
Add “Regulations Governing the central health competent authorities, the license holds
September Trace and track system for Medicinal or distributors shall establish information system for
6
product” tracing the source and tracking the flow of medicinal
procduct. In accordance with the regulations.
23