Page 30 - 2017食品藥物管理署年報(英文版)
P. 30
2017 Taiwan Food and Drug Administration Annual Report
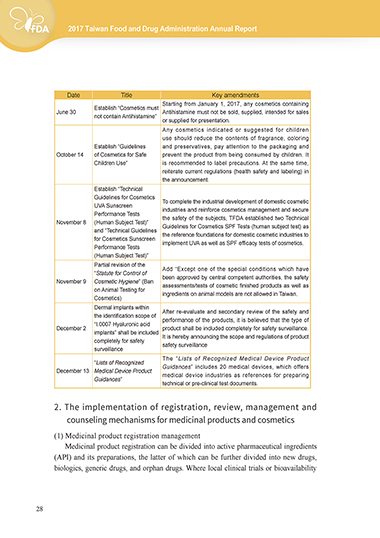
Date Title Key amendments
Starting from January 1, 2017, any cosmetics containing
Establish “Cosmetics must
June 30 Antihistamine must not be sold, supplied, intended for sales
not contain Antihistamine”
or supplied for presentation.
Any cosmetics indicated or suggested for children
use should reduce the contents of fragrance, coloring
Establish “Guidelines and preservatives, pay attention to the packaging and
October 14 of Cosmetics for Safe prevent the product from being consumed by children. It
Children Use” is recommended to label precautions. At the same time,
reiterate current regulations (health safety and labeling) in
the announcement.
Establish “Technical
Guidelines for Cosmetics To complete the industrial development of domestic cosmetic
UVA Sunscreen industries and reinforce cosmetics management and secure
Performance Tests the safety of the subjects, TFDA established two Technical
November 8 (Human Subject Test)” Guidelines for Cosmetics SPF Tests (human subject test) as
and “Technical Guidelines the reference foundations for domestic cosmetic industries to
for Cosmetics Sunscreen implement UVA as well as SPF efficacy tests of cosmetics.
Performance Tests
(Human Subject Test)”
Partial revision of the Add “Except one of the special conditions which have
“Statute for Control of been approved by central competent authorities, the safety
November 9 Cosmetic Hygiene” (Ban assessments/tests of cosmetic finished products as well as
on Animal Testing for ingredients on animal models are not allowed in Taiwan.
Cosmetics)
Dermal implants within
the identification scope of After re-evaluate and secondary review of the safety and
“I.0007 Hyaluronic acid performance of the products, it is believed that the type of
December 2 product shall be included completely for safety surveillance.
implants” shall be included
completely for safety It is hereby announcing the scope and regulations of product
surveillance safety surveillance
The “Lists of Recognized Medical Device Product
“Lists of Recognized
December 13 Medical Device Product Guidances” includes 20 medical devices, which offers
Guidances” medical device industries as references for preparing
technical or pre-clinical test documents.
2. The implementation of registration, review, management and
counseling mechanisms for medicinal products and cosmetics
(1) Medicinal product registration management
Medicinal product registration can be divided into active pharmaceutical ingredients
(API) and its preparations, the latter of which can be further divided into new drugs,
biologics, generic drugs, and orphan drugs. Where local clinical trials or bioavailability
28