Page 20 - 2017食品藥物管理署年報(英文版)
P. 20
2017 Taiwan Food and Drug Administration Annual Report
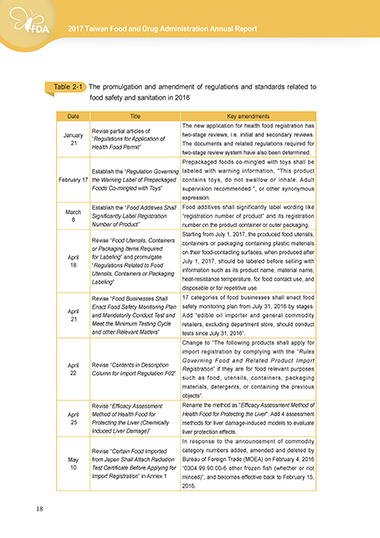
Table 2-1cThe promulgation and amendment of regulations and standards related to
food safety and sanitation in 2016
Date Title Key amendments
The new application for health food registration has
Revise partial articles of
January “Regulations for Application of two-stage reviews, i.e. initial and secondary reviews.
21 The documents and related regulations required for
Health Food Permit”
two-stage review system have also been determined.
Prepackaged foods co-mingled with toys shall be
Establish the “Regulation Governing labeled with warning information, "This product
February 17 the Warning Label of Prepackaged contains toys, do not swallow or inhale. Adult
Foods Co-mingled with Toys” supervision recommended ", or other synonymous
expression.
Establish the “Food Additives Shall Food additives shall significantly label wording like
March Significantly Label Registration “registration number of product” and its registration
8
Number of Product” number on the product container or outer packaging.
Starting from July 1, 2017, the produced food utensils,
Revise “Food Utensils, Containers containers or packaging containing plastic materials
or Packaging Items Required on their food-contacting surfaces, when produced after
April for Labeling” and promulgate July 1, 2017, should be labeled before selling with
18 “Regulations Related to Food
Utensils, Containers or Packaging information such as its product name, material name,
Labeling” heat-resistance temperature, for food contact use, and
disposable or for repetitive use.
Revise “Food Businesses Shall 17 categories of food businesses shall enact food
Enact Food Safety Monitoring Plan safety monitoring plan from July 31, 2016 by stages.
April and Mandatorily Conduct Test and Add “edible oil importer and general commodity
21
Meet the Minimum Testing Cycle retailers, excluding department store, should conduct
and other Relevant Matters” tests since July 31, 2016”.
Change to “The following products shall apply for
import registration by complying with the “Rules
Governing Food and Related Product Import
April Revise “Contents in Description Registration” if they are for food relevant purposes
22 Column for Import Regulation F02”
such as food, utensils, containers, packaging
materials, detergents, or containing the previous
objects”.
Revise “Efficacy Assessment Rename the method as "Efficacy Assessment Method of
April Method of Health Food for Health Food for Protecting the Liver". Add 4 assessment
25 Protecting the Liver (Chemically methods for liver damage-induced models to evaluate
Induced Liver Damage)” liver protection effects.
In response to the announcement of commodity
Revise “Certain Food Imported category numbers added, amended and deleted by
May from Japan Shall Attach Radiation Bureau of Foreign Trade (MOEA) on February 4, 2016
10 Test Certificate Before Applying for “0304.99.90.00-6 other frozen fish (whether or not
Import Registration” in Annex 1 minced)”, and becomes effective back to February 15,
2016.
18