Page 29 - 2017食品藥物管理署年報(英文版)
P. 29
2017 Taiwan Food and Drug Administration Annual Report Chapter 2. Legal Environment and Registration
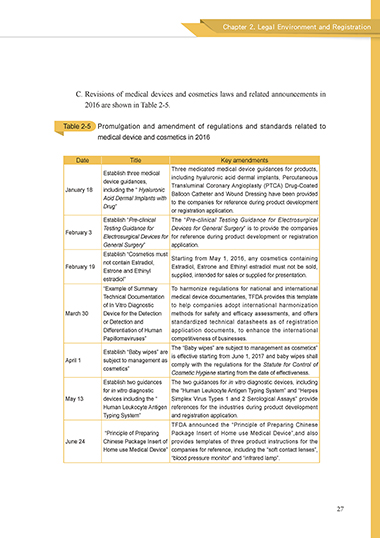
C. Revisions of medical devices and cosmetics laws and related announcements in
2016 are shown in Table 2-5.
Table 2-5 Promulgation and amendment of regulations and standards related to
medical device and cosmetics in 2016
Date Title Key amendments
Three medicated medical device guidances for products,
Establish three medical
device guidances, including hyaluronic acid dermal implants, Percutaneous
January 18 including the “ Hyaluronic Transluminal Coronary Angioplasty (PTCA) Drug-Coated
Acid Dermal Implants with Balloon Catheter and Wound Dressing have been provided
Drug” to the companies for reference during product development
or registration application.
Establish “Pre-clinical The “Pre-clinical Testing Guidance for Electrosurgical
Testing Guidance for Devices for General Surgery” is to provide the companies
February 3
Electrosurgical Devices for for reference during product development or registration
General Surgery” application.
Establish “Cosmetics must Starting from May 1, 2016, any cosmetics containing
not contain Estradiol,
February 19 Estradiol, Estrone and Ethinyl estradiol must not be sold,
Estrone and Ethinyl supplied, intended for sales or supplied for presentation.
estradiol”
“Example of Summary To harmonize regulations for national and international
Technical Documentation medical device documentaries, TFDA provides this template
of In Vitro Diagnostic to help companies adopt international harmonization
March 30 Device for the Detection methods for safety and efficacy assessments, and offers
or Detection and standardized technical datasheets as of registration
Differentiation of Human application documents, to enhance the international
Papillomaviruses” competitiveness of businesses.
The “Baby wipes” are subject to management as cosmetics”
Establish “Baby wipes” are is effective starting from June 1, 2017 and baby wipes shall
April 1 subject to management as comply with the regulations for the Statute for Control of
cosmetics”
Cosmetic Hygiene starting from the date of effectiveness.
Establish two guidances The two guidances for in vitro diagnostic devices, including
for in vitro diagnostic the “Human Leukocyte Antigen Typing System” and “Herpes
May 13 devices including the “ Simplex Virus Types 1 and 2 Serological Assays” provide
Human Leukocyte Antigen references for the industries during product development
Typing System” and registration application.
TFDA announced the “Principle of Preparing Chinese
“Principle of Preparing Package Insert of Home use Medical Device”,and also
June 24 Chinese Package Insert of provides templates of three product instructions for the
Home use Medical Device” companies for reference, including the “soft contact lenses”,
“blood pressure monitor” and “infrared lamp”.
27