Page 26 - 2017食品藥物管理署年報(英文版)
P. 26
2017 Taiwan Food and Drug Administration Annual Report
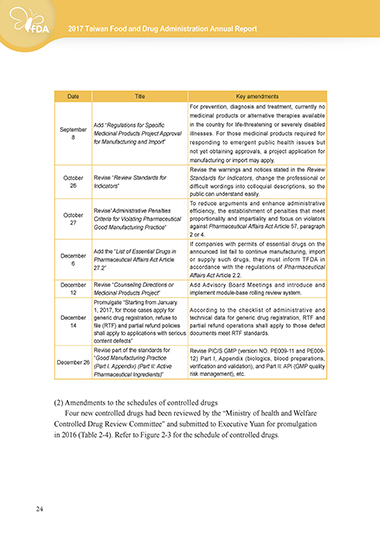
Date Title Key amendments
For prevention, diagnosis and treatment, currently no
medicinal products or alternative therapies available
Add “Regulations for Specific in the country for life-threatening or severely disabled
September Medicinal Products Project Approval illnesses. For those medicinal products required for
8
for Manufacturing and Import” responding to emergent public health issues but
not yet obtaining approvals, a project application for
manufacturing or import may apply.
Revise the warnings and notices stated in the Review
October Revise “Review Standards for Standards for Indicators, change the professional or
26 Indicators” difficult wordings into colloquial descriptions, so the
public can understand easily.
To reduce arguments and enhance administrative
Revise“Administrative Penalties efficiency, the establishment of penalties that meet
October Criteria for Violating Pharmaceutical proportionality and impartiality and focus on violators
27
Good Manufacturing Practice” against Pharmaceutical Affairs Act Article 57, paragraph
2 or 4.
If companies with permits of essential drugs on the
Add the “List of Essential Drugs in announced list fail to continue manufacturing, import
December Pharmaceutical Affairs Act Article or supply such drugs, they must inform TFDA in
6
27.2” accordance with the regulations of Pharmaceutical
Affairs Act Article 2.2.
December Revise “Counseling Directions or Add Advisory Board Meetings and introduce and
12 Medicinal Products Project” implement module-base rolling review system.
Promulgate “Starting from January
1, 2017, for those cases apply for According to the checklist of administrative and
December generic drug registration, refuse to technical data for generic drug registration, RTF and
14 file (RTF) and partial refund policies partial refund operations shall apply to those defect
shall apply to applications with serious documents meet RTF standards.
content defects”
Revise part of the standards for Revise PIC/S GMP (version NO. PE009-11 and PE009-
“Good Manufacturing Practice 12) Part I, Appendix (biologics, blood preparations,
December 26
(Part I. Appendix) (Part II: Active verification and validation), and Part II: API (GMP quality
Pharmaceutical Ingredients)” risk management), etc.
(2) Amendments to the schedules of controlled drugs
Four new controlled drugs had been reviewed by the “Ministry of health and Welfare
Controlled Drug Review Committee” and submitted to Executive Yuan for promulgation
in 2016 (Table 2-4). Refer to Figure 2-3 for the schedule of controlled drugs.
24