Page 22 - 2017食品藥物管理署年報(英文版)
P. 22
2017 Taiwan Food and Drug Administration Annual Report
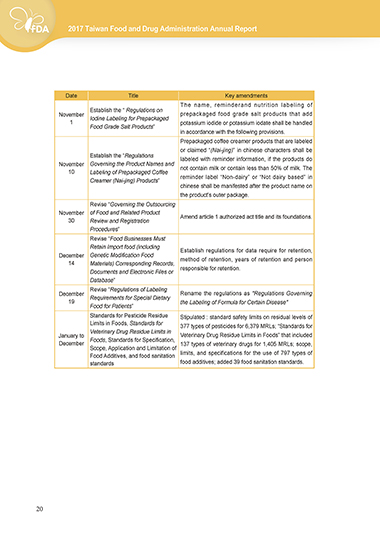
Date Title Key amendments
The name, reminderand nutrition labeling of
Establish the “ Regulations on
November Iodine Labeling for Prepackaged prepackaged food grade salt products that add
1 potassium iodide or potassium iodate shall be handled
Food Grade Salt Products”
in accordance with the following provisions.
Prepackaged coffee creamer products that are labeled
or claimed “(Nai-jing)” in chinese characters shall be
Establish the “Regulations labeled with reminder information, if the products do
November Governing the Product Names and not contain milk or contain less than 50% of milk. The
10 Labeling of Prepackaged Coffee
Creamer (Nai-jing) Products” reminder label “Non-dairy” or “Not dairy based” in
chinese shall be manifested after the product name on
the product's outer package.
Revise “Governing the Outsourcing
November of Food and Related Product Amend article 1 authorized act title and its foundations.
30 Review and Registration
Procedures”
Revise “Food Businesses Must
Retain Import food (including Establish regulations for data require for retention,
December Genetic Modification Food method of retention, years of retention and person
14 Materials) Corresponding Records,
Documents and Electronic Files or responsible for retention.
Database”
Revise “Regulations of Labeling
December Requirements for Special Dietary Rename the regulations as "Regulations Governing
19 the Labeling of Formula for Certain Disease"
Food for Patients”
Standards for Pesticide Residue Stipulated : standard safety limits on residual levels of
Limits in Foods, Standards for 377 types of pesticides for 6,379 MRLs; “Standards for
Veterinary Drug Residue Limits in
January to Veterinary Drug Residue Limits in Foods” that included
December Foods, Standards for Specification, 137 types of veterinary drugs for 1,405 MRLs; scope,
Scope, Application and Limitation of
Food Additives, and food sanitation limits, and specifications for the use of 797 types of
standards food additives; added 39 food sanitation standards.
20