
2015 Annual Report
85
Risk Assessment
Management and
Research Outcomes
Cosmetics
Management
Appendix
National
Laboratory and
Testing Network
Risk Communication
and Consumer
Protection
International
Cooperation and
Cross-Strait Exchange
Food
Management
Medicinal
Products
Management
Policy and
Organization
Controlled
Drugs
Management
Medical
Devices
Management
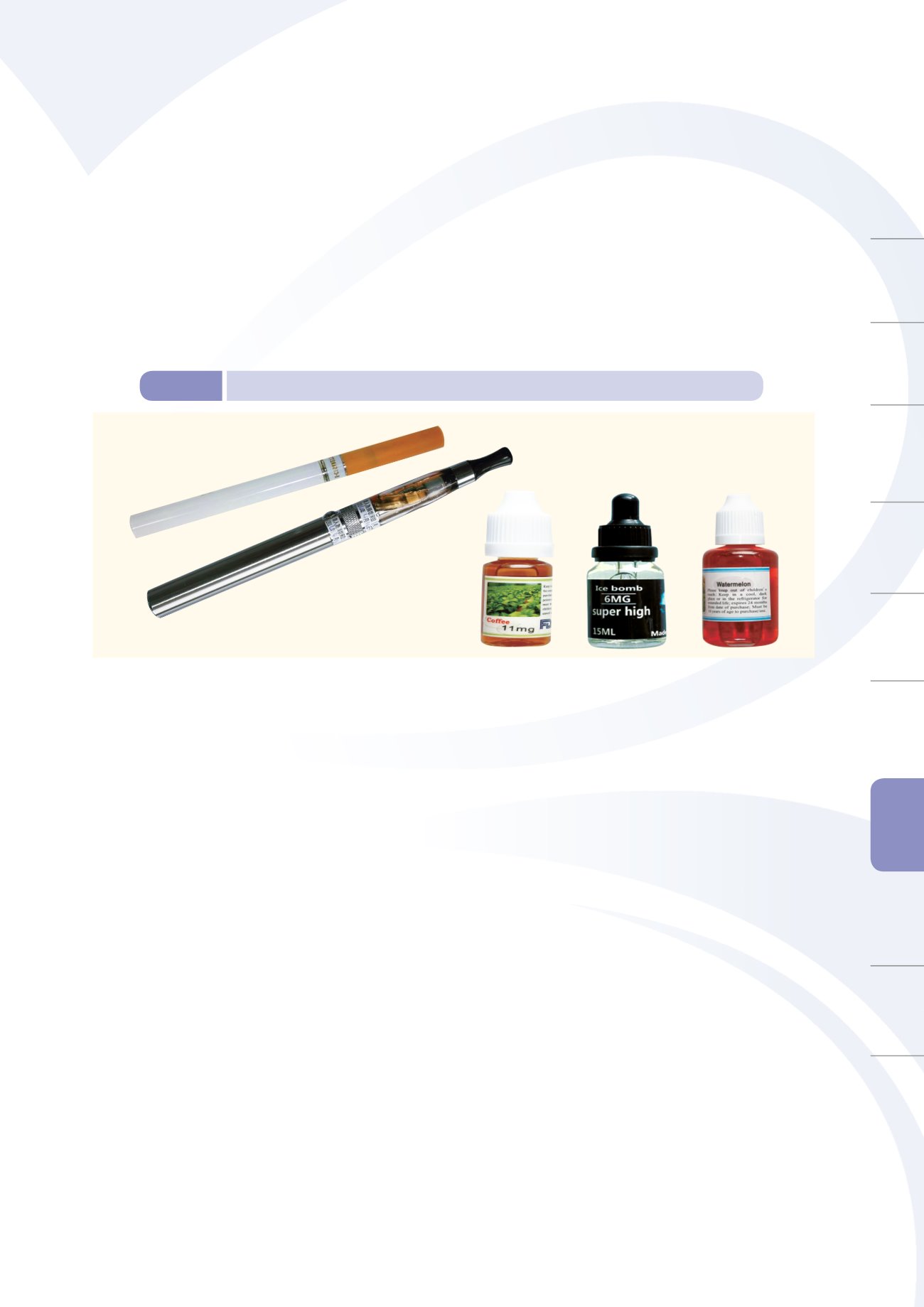
b. TFDA therefore analyzed a total of 31 samples of electronic cigarette replenishment fluid (shown
in Figure 8-4), of which seven contained nicotine, 28 contained acetaldehyde and 31 contained
formaldehyde. A press release was issued to explain the 2014 test results for nicotine contents in
electronic cigarettes, its impacts to health, and related laws and regulations.
(9) Detection of
Pseudomonas aeruginosa
and Preservatives in Shower Foam
a. In July 2014, the Consumer Protection Committee of the Executive Yuan conducted microbial
content and labeling inspections for shower foam, shampoo, and other products from 16 hotels
and inns. Results showed that seven hotels and inns failed to comply with the regulations, including
labeling violations as well as one case of aerobic plate count exceeding limits and the presence of
Pseudomonas aeruginosa
.
b. To strengthen the hygiene management of sanitation products in the hospitality industry, TFDA
notified local health bureaus to conduct sample testing. Test results showed that aerobic
plate count from three products exceeded the
Permitted Standards for Microbial Content in
Cosmetics
proclaimed by TFDA. These three products were transferred to the responsible
health bureaus to conduct recalls and scrapping within a specified deadline.
c. The 402
nd
issue of the consumer report published in October 2014 included an article entitled
46% of Shower Foam Tested Positive for Preservatives
. TFDA immediately requested local
health bureaus to collect samples of the marketed products.
d. Test results showed that preservative contents are compliant to the
Standards on Preservative
Contents, Usage, and Limits for Cosmetics.
Products with nonconforming labels were
transferred to the responsible health bureaus for handling.
(10) Type A Botulinum Toxin Preparations Imported Without Permit
a. The demand for medical beauty is growing tremendously in Taiwan. Illegal imports of Type A
botulinum toxin preparations are common place. Thus, TFDA continued to assist the Justice
authority to identify whether preparations contain type A botulinum toxin.
b. To eliminate illegal imports of botulinum toxin preparations and other biological medication,
TFDA held a press conference entitled
Beauty Reminders: Three Simple Tips to Identify Legal
Botox Preparations
to provide the public with simple steps to recognize the legal drugs.
Figure 8-4
Visual appearance of an electronic cigarette specimen



















