
Food and Drug Administration
88
Policies and Outcomes
1. Accreditation for Food as well as Drugs and Cosmetics Laboratories
(1) Expanding Laboratory Testing Capabilities
a. Accreditation systems for food laboratories and drugs and cosmetics laboratories were initiated in
2004 and 2008 respectively. In 2010, laboratory accreditation action plans were further expanded
and accelerated to quickly increase testing capabilities through continued implementation of border
inspections of food and Traditional Chinese Medicine (TCM) products, establishment of standard
limits for TCM, and accreditation of test items outsourced by administrative processes.
b. The number of accredited laboratories and tests increased from 36 laboratories and 353 tests in
2009 to 91 laboratories and 1,153 tests in 2014. These laboratories included 61 accredited food
testing laboratories and 30 accredited drugs and cosmetics testing laboratories with 48, 13, and 30
laboratories in northern, central, and southern Taiwan respectively.
(2) Strengthened Supervision and Management Systems
a. Regular and Unannounced Audits
To effectively supervise and manage accredited laboratories and to establish a system for verifying
the reliability of test data, TFDA performed a total of 108 audits, of which 88 were first-time audits,
audits for increases or changes in testing scope, extension audits, and surveillance audits as well
as 20 unannounced audits.
b. Organizing Proficiency Tests
Proficiency test results of various accredited laboratories are publicly released on a regular basis.
In 2014, a total of 26 proficiency tests (20 for food and 6 for drugs and cosmetics) were held. Of
which, 2 laboratories had their accreditation revoked for failure to meet proficiency test standards.
A total of 4
Laboratory Accreditation Test Using Double-Blind Samples
were also used to verify the
correctness of test data.
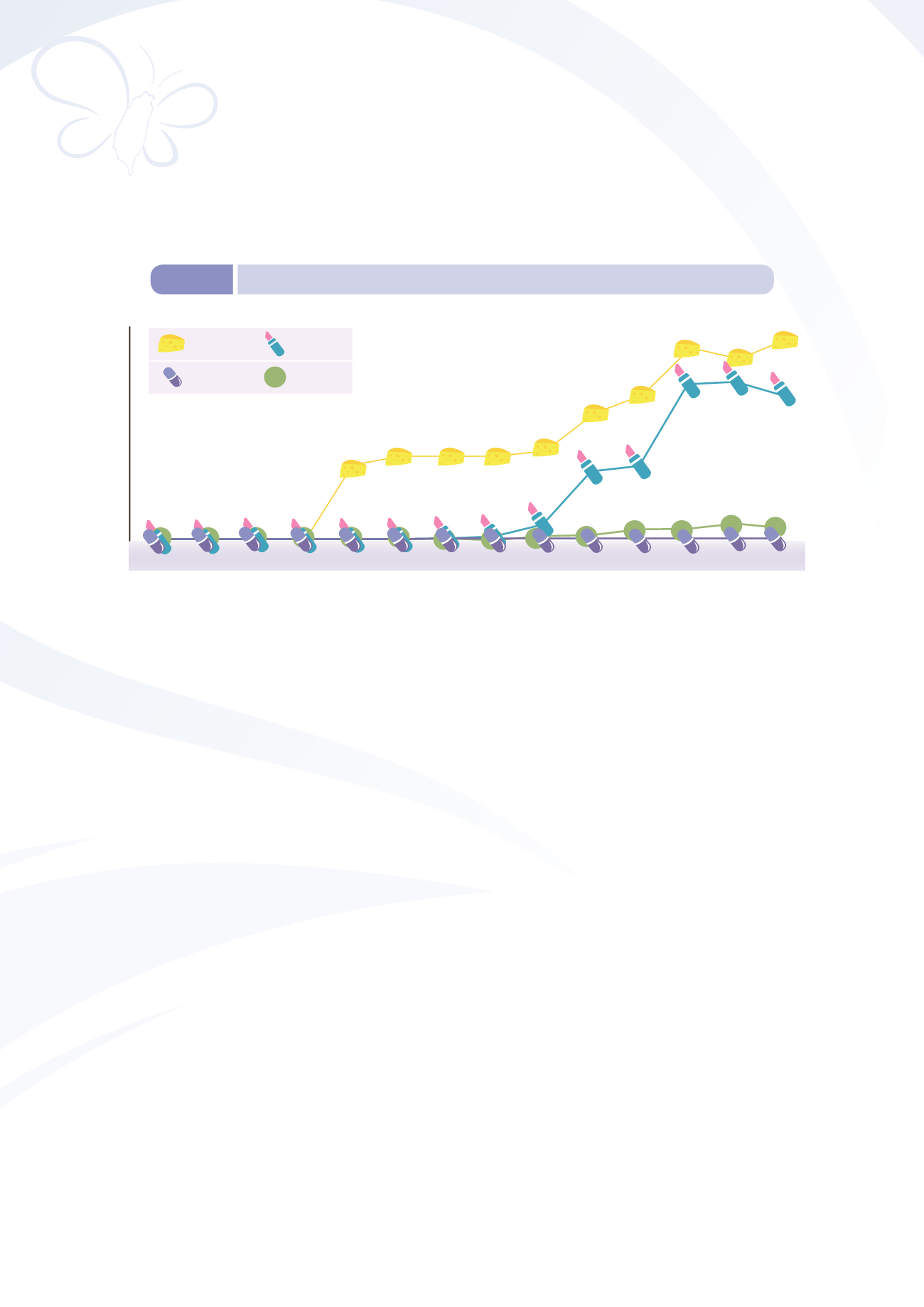
Figure 8-7
Number of accredited tests over time
7
7
7
7
7
7
9
9
9
9
9
9
9
9
525
488
517
248
230
55
16
39
58 56
20
17
41
3
280
280 280 298
421
481
637
600
665
0
100
200
300
400
500
600
700
GLP
GLP
GLP
GLP
GLP
GLP
GLP
GLP
GLP
GLP
GLP
GLP
GLP GLP
GLP
Foods
Drug Abuse
GLP
Drugs
and Cosmetics
Year
2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014
Accredited laboratories



















