
2015 Annual Report
91
Risk Assessment
Management and
Research Outcomes
Cosmetics
Management
Appendix
National
Laboratory and
Testing Network
Risk Communication
and Consumer
Protection
International
Cooperation and
Cross-Strait Exchange
Food
Management
Medicinal
Products
Management
Policy and
Organization
Controlled
Drugs
Management
Medical
Devices
Management
10 laboratory audits completed. 1 Dimethyl Yellow and Diethyl Yellow
test proficiency test was carried out.
Private laboratories publicly announced: 10 laboratories ;
private laboratories test capabilities: 425 cases/day
4 laboratory audits completed.
1 benzopyrene test proficiency test was carried out.
Private laboratories publicly announced: 4 laboratories ;
private laboratories test capabilities: 90 cases/day
September
December
2014
2014
Tainted Lard incident
Dimethyl Yellow and Diethyl Yellow Event
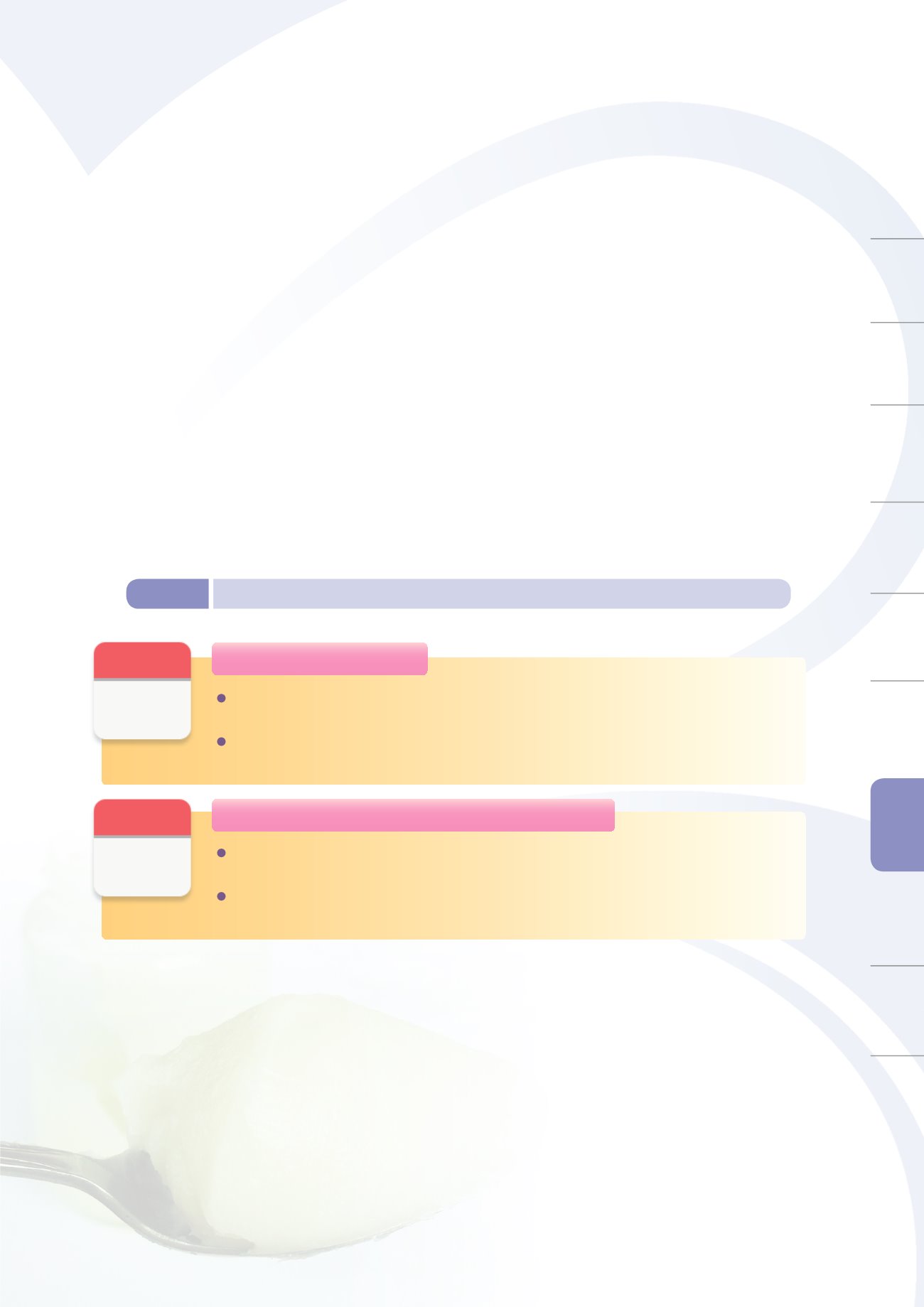
Figure 8-8
Private laboratory mobilization outcomes in response to emergency incidents in 2014
3. Outcomes of Emergency Testing Mobilization
(1) List of Laboratories and Testing Capabilities
Given the large demand for testing, the TFDA has surveyed the
National Inspection Resource Database
to identify private institutions with the necessary testing instruments and capacity to participate in
emergency testing. In response to emergency incidents from 2013 to 2014 such as maleic anhydride
modified starch, tainted oil, adulterated lard, and Dimethyl Yellow, private laboratories have been
mobilized to provide testing services. Figure 8-8 shows the results of such mobilization.
(2) Quality Management of Laboratory Testing
As a result of emergencies, testing quality of the laboratories publicly listed by the TFDA is
managed via documentation and data reviews, on-site audits, and proficiency testing in order to
thoroughly assess the testing capabilities of the laboratories. Any laboratory that fails to pass on-
site audits or proficiency testing is
prohibited from receiving samples for the specified tests.



















