
Food and Drug Administration
10
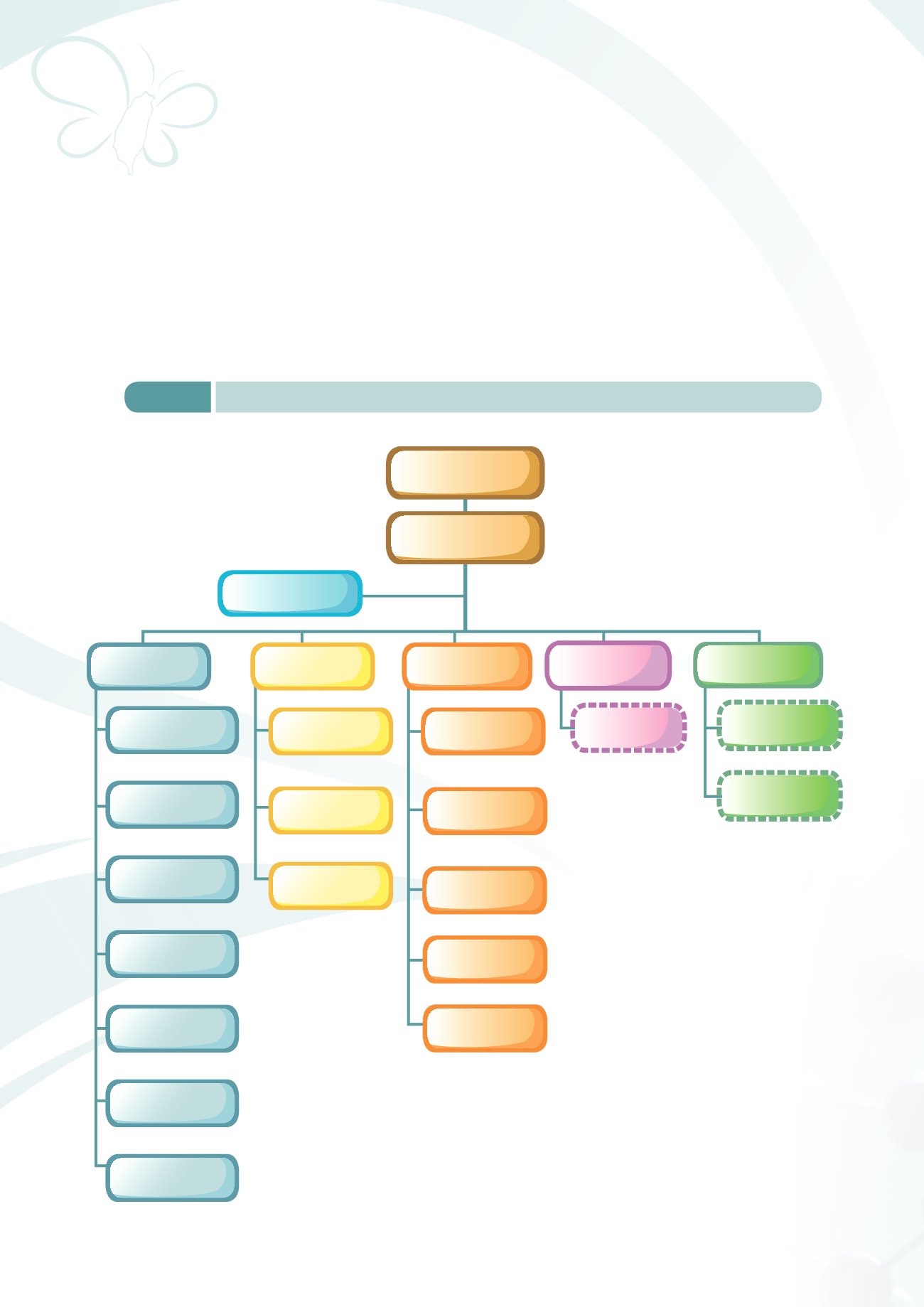
Figure 1-1
Organization chart of, Food and Drug Administration, Ministry of Health and Welfare
產品及法規管理
食品組
藥品組
產品及法規管理
Chief Secretary
Deputy Director-General
Director-General
Northern Center
for Regional
Administration
Central Center
for Regional
Administration
Southern Center
for Regional
Administration
Division of Planning and
Research Development
Division of Food Safety
Division of
Medicinal Products
Division of Medical
Devices and Cosmetics
Division of
Controlled Drugs
Division of
Research & Analysis
Division of
Risk Management
Division
Regional Center
Administration Office
Task Force Unit
Factory for
Controlled Drugs
Collaborative Partner
Center for
Drug Evaluation
Taiwan Drug
Relief Foundation
Office of Secretariat
Office of Personnel
Office of Accounting
Office of
Service Ethics
Office of
Information Management
3. At the merger of 2010, TFDA had a personnel headcount of 467 individuals. In 2011, the
task of border inspections was handed over from the Ministry of Economic Affairs Bureau of
Standards, Metrology and Inspection to TFDA, raising personnel headcount by 18 individuals
to a total of 485. However, the headcount was reduced in 2012 to 484 to reduce redundant
personnel. In 2013, in response to organizational restructuring and the need to carry out
relevant businesses, the headcount was further increased to 527 individuals. In 2014, TFDA
headcount was once again raised to 602 individuals after approval by the Executive Yuan.



















