Page 121 - 2018食藥署年報(英文版)
P. 121
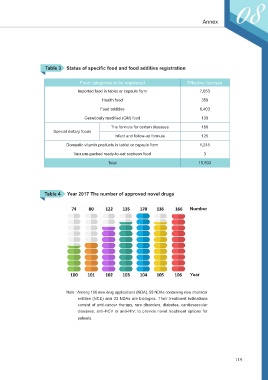
Table 3 Status of specific food and food additive registration
Food categories to be registered Effective licenses
Imported food in tablet or capsule form 7,053
Health food 358
Food additive 6,403
Genetically modified (GM) food 130
The formula for certain diseases 186
Special dietary foods
Infant and follow-up formula 125
Domestic vitamin products in tablet or capsule form 1,245
Vacuum-packed ready-to-eat soybean food 3
Total 15,503
Table 4 Year 2017 The number of approved novel drugs
Number
Year
Note : Among 166 new drug applications (NDA), 58 NDAs containing new chemical
entities (NCE) and 33 NDAs are biologics. Their treatment indications
consist of anti-cancer therapy, rare disorders, diabetes, cardiovascular
diseases, anti-HCV or anti-HIV, to provide novel treatment options for
patients.
119