Page 122 - 2018食藥署年報(英文版)
P. 122
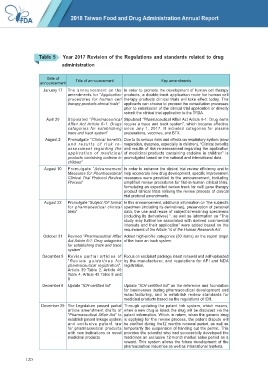
Table 5 Year 2017 Revision of the Regulations and standards related to drug
administration
Date of
announcement Title of announcement Key amendments
January 17 The announcement on the In order to promote the development of human cell therapy
amendments for “Application products, a double-track application route for human cell
procedures for human cell therapy products clinical trials will take effect today. The
therapy products clinical trials” applicants can choose to proceed the consultation processes
prior to submission of the clinical trial application or directly
submit the clinical trial application to the TFDA.
April 20 Stipulated “Pharmaceutical Stipulated “Pharmaceutical Affair Act Article 6-1: Drug items
Affair Act Article 6-1: Drugs require a trace and track system”, which became effective
catagories for establishing since July 1, 2017. It included categories for plasma
trace and track system” preparations, vaccines, and BTX.
August 2 Promulgate “Clinical benefits Due to its serious risks and effects on respiratory system (slow
and results of risk re- respiration, dyspnea, especially in children), “Clinical benefits
assessment regarding the and results of risk re-assessment regarding the application
application of medicinal of medicinal products containing codeine in children” is
products containing codeine in promulgated based on the national and international data.
children”
August 10 Promulgate “Advancement In order to enhance the clinical trial review efficiency and to
Measures for Pharmaceutical help accelerate new drug development, specific improvement
Clinical Trial Protocol Review measures were provided in the announcement, including
Process” simplified review procedures for first-in-human clinical trials,
formulating an expedited review track for cell/ gene therapy
product clinical trials refining the review process of clinical
trial protocol amendments.
August 22 Promulgate “Subject ICF format In this announcement, additional information on “the subject's
for pharmaceutical clinical specimen (including its derivatives), preservation of personal
trials” data, the use and reuse of subject's/remaining specimens
(including its derivatives) ”, as well as information on “This
study may further be associated with derived commercial
interests and their application” were added based on the
requirement of the Article 14 of the Human Research Act .
October 31 Revised “Pharmaceutical Affair Added high-profile categories (30 items) as the report target
Act Article 6-1: Drug catagories of the trace an track system.
for establishing track and trace
system”
December 5 Revise partial articles of Focus on excipient package insert renewal and self-uploaded
“ Review guidelines for by the manufacturer, and regulations for API and NDA
pharmaceutical registration”: registration.
Article 39 Table 2, Article 40
Table 4, Article 42 Table 8 and
9
December 6 Update “ICH-certified list” Update “ICH-certified list” as the reference and foundation
for businesses during pharmaceutical development and
manufacturing, and to establish review standards for
medicinal products based on the regulations of ICH.
December 29 The Legislature passed partial Through updating the patent link system, which means,
article amendment drafts of when a new drug is listed, the drug will be disclosed via the
“Pharmaceutical Affair Act” to patent information. Which in return, when the generic drug
establish patent linkage system is applying for the review process, the patent dispute will
and exclusive patent law be clarified during the12 months renewal period, as well as
for pharmaceutical products temporarily the suspension of handing out the permit. This
with new indications or novel provides the scientist who had successfully developed the
medicinal products medicines an exclusive 12-month market sales period as a
reward. This system allows the future development of the
pharmaceutical industries as well as international markets.
120