Page 126 - Taiwan Food and Drug Administration 2016 Annual Report
P. 126
Taiwan Food and Drug Adminstration
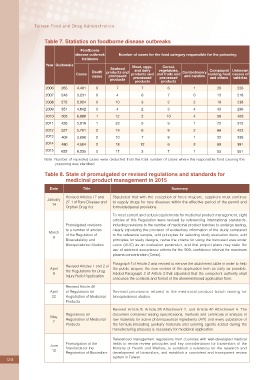
Table 7. Statistics on foodborne disease outbreaks
Foodborne
disease outbreak Number of cases for the food category responsible for the poisoning
incidence
Year Outbreaks Meat, eggs, Cereal,
Seafood
Death products and and dairy vegetables, Confeetionery Compound Unknown
Cases products and and fruits and cooking food causes of
cases processed processed processed and candies and others vehicles
products
products products
2006 265 4,401 0 7 7 6 1 20 226
2007 248 3,231 0 4 6 7 0 13 218
2008 272 2,924 0 10 3 2 2 19 236
2009 351 4,642 0 4 2 3 4 43 296
2010 503 6,880 1 12 2 10 4 56 420
2011 426 5,819 1 23 5 9 1 73 315
2012 527 5,701 0 19 8 9 2 66 423
2013 409 3,890 0 10 7 9 1 22 338
2014 480 4,504 0 18 12 6 3 60 381
2015 632 6,235 0 17 3 7 1 53 551
Note: Number of repeated cases were deducted from the total number of cases where the responsible food causing the
poisoning was identi?ed
Table 8. State of promulgated or revised regulations and standards for
medicinal product management in 2015
Date Title Summary
Revised Articles 17 and Stipulated that with the exception of force majeure, suppliers must continue
January 27-1 of Rare Disease and to supply drugs for rare diseases within the effective period of the permit and
14
Orphan Drug Act formulatedpenal provisions.
To meet current and actual requirements for medicinal product management, eight
articles of this Regulation were revised by referencing international standards,
Promulgated revisions including revisions to the number of medicinal product batches to undergo testing,
to a number of articles clearly stipulating the provision of evidentiary information of the study compared
March
of the Regulation of to the reference sample, add principles for selecting study evaluation items, add
9
Bioavailability and principles for study designs, revise the criteria for using the truncated area under
Bioequivalence Studies curve (AUC) as an evaluation parameter, and that project plans may state the
use of widened acceptance criteria for the 90% con?dence interval for maximum
plasma concentration (Cmax).
Paragraph 1of Article 2 was revised to remove the attachment table in order to help
Revised Articles 1 and 2 of
April the public acquire the new version of the application form as early as possible.
the Regulations for Drug
9 Added Paragraph 2 of Article 2 that stipulated that the competent authority shall
Injury Relief Application
announce the contents and format of the aforementioned application form.
Revised Article 46
April of Regulations for Revised provisions related to the medicinal product batch testing for
22 Registration of Medicinal bioequivalence studies.
Products
Revised Article 9, Article 39 Attachment 2, and Article 40 Attachment 4. The
Regulations for document contained testing speci?cations, methods and certi?cate of analysis of
May
Registration of Medicinal raw materials for active pharmaceutical ingredients (API) and every substance of
7
Products the formula (including auxiliary materials and coloring agents added during the
manufacturing process) is necessary for medicinal application.
Referenced management regulations from countries with well-developed medical
Promulgation of the fields to revise review principles and key considerations for biosimilars of the
June
Standards for the Ministry of Health and Welfare, to establish a reference for the research and
12
Registration of Biosimilars development of biosimilars, and establish a consistent and transparent review
system in Taiwan.
124