Page 129 - Taiwan Food and Drug Administration 2016 Annual Report
P. 129
2016 ANNUAL
REPORT
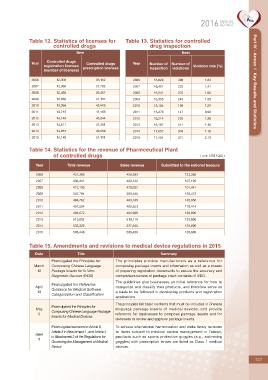
Table 12. Statistics of licenses for Table 13. Statistics for controlled
controlled drugs drug inspection
Item Item
Controlled drugs
Year Controlled drugs Year Number of Number of
registration licenses Violation rate (%)
prescription licenses inspection violations
(number of licenses)
2006 12,302 36,112 2006 16,629 306 1.84 Part IV - Annex 1 Key Results and Statistics
2007 12,360 37,792 2007 16,451 232 1.41
2008 12,465 39,467 2008 16,241 270 1.66
2009 12,830 41,157 2009 16,355 245 1.50
2010 13,266 42,619 2010 15,154 196 1.29
2011 13,745 44,469 2011 15,270 147 0.96
2012 14,149 45,844 2012 16,214 202 1.25
2013 14,511 47,391 2013 16,197 211 1.30
2014 14,857 49,059 2014 17,057 304 1.78
2015 15,148 51,111 2015 17,454 371 2.13
Table 14. Statistics for the revenue of Pharmceutical Plant
of controlled drugs ( unit: NT$ 1000 )
Year Total revenue Sales revenue Submitted to the national treasury
2006 431,369 426,393 123,385
2007 436,341 433,122 107,105
2008 477,133 470,627 101,441
2009 507,794 505,340 138,473
2010 484,762 483,169 145,956
2011 491,524 489,523 116,414
2012 494,672 491,909 120,000
2013 513,092 510,119 120,000
2014 533,320 527,940 120,000
2015 593,448 586,406 120,000
Table 15. Amendments and revisions to medical device regulations in 2015
Date Title Summary
Promulgated the Principles for The principles provide manufacturers as a reference for
March Composing Chinese Language composing package inserts and information as well as a means
12 Package Inserts for In Vitro of preparing registration documents to ensure the accuracy and
Diagnostic Devices (IVDD) comprehensiveness of package insert contents of IVDD.
The guidelines give businesses an initial reference for how to
Promulgated the Reference
April categorize and classify their products, and therefore serve as
Guidance for Medical Software
13 a basis to be followed in developing products and registration
Categorization and Classification
applications.
The principles list basic contents that must be included in Chinese
Promulgated the Principles for
May language package inserts of medical devices, and provide
Composing Chinese Language Package
5 reference for businesses to compose package inserts and for
Inserts for Medical Devices
reviewers to review and approve package inserts.
Promulgated revisions to Article 8, To achieve international harmonization and make timely revisions
Article 3 in Attachment 1, and Article 4 to items subject to medical device management in Taiwan,
June
in Attachment 2 of the Regulations for products such as sports protective goggles (e.g., swimming
3
Governing the Management of Medical goggles) with prescription lenses are listed as Class 1 medical
Device devices.
127