Page 122 - Taiwan Food and Drug Administration 2016 Annual Report
P. 122
Taiwan Food and Drug Adminstration
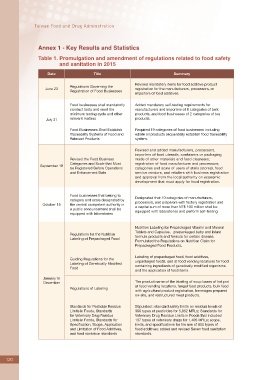
Annex 1 - Key Results and Statistics
Table 1. Promulgation and amendment of regulations related to food safety
and sanitation in 2015
Date Title Summary
Revised mandatory items for food additive product
Regulations Governing the
June 23 registration for the manufacturers, processors, or
Registration of Food Businesses
importers of food additives.
Food businesses shall mandatorily Added mandatory self-testing requirements for
conduct tests and meet the manufacturers and importers of 8 categories of bulk
minimum testing cycle and other products, and food businesses of 2 categories of tea
July 31 relevant matters products.
Food Businesses Shall Establish Required 19 categories of food businesses including
Traceability Systems of Food and edible oil products sequentially establish food traceability
Relevant Products system.
Revised and added manufacturers, processors,
importers of food utensils, containers or packaging
Revised the Food Business made of other materials and food cleansers;
Categories and Scale that Must registration of food manufacturer and processors;
September 18
be Registered Before Operations categories and scale of users of stalls (stores), food
and Enforcement Date service vendors, and retailers with business registration
and approval from the local authority on economic
development that must apply for food registration.
Food businesses that belong to Designated that 10 categories of manufacturers,
category and scale designated by
October 15 the central competent authority in processors, and preparers with factory registration and
a capital sum of more than NT$ 100 million shall be
a public announcement shall be equipped with laboratories and perform self-testing.
equipped with laboratories
Nutrition Labeling for Prepackaged Vitamin and Mineral
Tablets and Capsules, prepackaged baby and infant
Regulations for the Nutrition
formula products and formula for certain disease.
Labeling of Prepackaged Food
Formulated the Regulations on Nutrition Claim for
Prepackaged Food Products.
Labeling of prepackaged food, food additives,
Guiding Regulations for the
unpackaged foods, and at food vending locations for food
Labeling of Genetically Modified
containing ingredients of genetically modified organisms
Food
and the application of food items.
January to
December The product name of the labeling of soup bases of hot pot
at food vending locations, fungal food products, bulk food
Regulations of Labeling
with agricultural product registration, beverages prepared
on-site, and restructured meat products.
Standards for Pesticide Residue Stipulated : standard safety limits on residual levels of
Limits in Foods, Standards 366 types of pesticides for 5,852 MRLs; Standards for
for Veterinary Drug Residue Veterinary Drug Residue Limits in Foods that included
Limits in Foods, Standards for 137 types of veterinary drugs for 1,405 MRLs; scope,
Specification, Scope, Application limits, and specifications for the use of 800 types of
and Limitation of Food Additives, food additives; added and revised Seven food sanitation
and food sanitation standards standards.
120