Page 125 - Taiwan Food and Drug Administration 2016 Annual Report
P. 125
2016 ANNUAL
REPORT
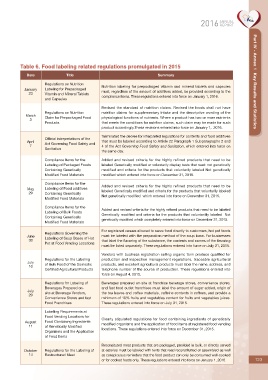
Table 6. Food labeling related regulations promulgated in 2015
Date Title Summary
Regulations on Nutrition Part IV - Annex 1 Key Results and Statistics
Nutrition labeling for prepackaged vitamin and mineral tablets and capsules
January Labeling for Prepackaged must, regardless of the amount of additives added, be provided according to the
23 Vitamin and Mineral Tablets
compliance items. These regulations entered into force on January 1, 2016.
and Capsules
Revised the standard of nutrition claims. Revised the foods shall not have
Regulations on Nutrition nutrition claims for supplementary intake and the descriptive wording of the
March Claim for Prepackaged Food physiological functions of nutrients. Where a product has two or more nutrients
3
Products that meets the conditions for nutrition claims, such claim may be made for such
product accordingly.These revisions entered into force on January 1, 2016.
Terminated the decree for interpreted regulations for contents and food additives
Official interpretations of the
April Act Governing Food Safety and that must be labeled according to Article 22 Paragraph 1 Subparagraphs 2 and
7 4 of the Act Governing Food Safety and Sanitation, which entered into force on
Sanitation
the same day.
Compliance Items for the Added and revised criteria for the highly refined products that need to be
Labeling of Packaged Foods labeled Genetically modified or voluntarily display tests that read: not genetically
Containing Genetically modified and criteria for the products that voluntarily labeled Not genetically
Modified Food Materials modified which entered into force on December 31, 2015.
Compliance Items for the
Added and revised criteria for the highly refined products that need to be
May Labeling of Food additives
29 Containing Genetically labeled Genetically modified and criteria for the products that voluntarily labeled
Not genetically modified which entered into force on December 31, 2015.
Modified Food Materials
Compliance Items for the
Added and revised criteria for the highly refined products that need to be labeled
Labeling of Bulk Foods
Genetically modified and criteria for the products that voluntarilily labeled Not
Containing Genetically
genetically modified which completely entered into force on December 31, 2015.
Modified Food Materials
For registered venues allowed to serve food directly to customers, hot pot foods
Regulations Governing the
June Labeling of Soup Bases of Hot must be labeled with the preparation method of the soup base. For businesses
30 that label the flavoring of the substance, the contents and names of the flavoring
Pot at Food Vending Locations
must be listed separately. These regulations entered into force on July 31, 2015.
Vendors with business registration selling organic farm produce qualified for
Regulations for the Labeling production and inspection management regulations, traceable agricultural
July of Bulk Food of the Domestic products, and excellent agricultural products must label the name, address, and
10
Certified Agricultural Products telephone number of the source of production. These regulations entered into
force on August 4, 2015.
Regulations for Labeling of Beverages prepared on-site at franchise beverage stores, convenience stores,
Beverages Prepared on- and fast food outlet franchises must label the amount of sugar added, origin of
July
20 site at Beverage Vendors, the tea leaves and coffee materials, caffeine contents in coffees, and provide a
Convenience Stores and fast minimum of 10% fruits and vegetables content for fruits and vegetables juices.
Food Franchises These regulations entered into force on July 31, 2015.
Labelling Requirements at
Food Vending Locations for
Clearly stipulated regulations for food containing ingredients of genetically
August Food Containing Ingredients modified organisms and the application of food items at registered food vending
11 of Genetically Modified
locations. These regulations entered into force on December 31, 2015.
Organisms and the Application
of Food Items
Reconsituted meat products that are packaged, provided in bulk, or directly served
October Regulations for the Labeling of at eateries must be labeled with texts that read reconstitutted or assembled as well
14 Restructured Meat as conspicuous reminders that the food product can only be consumed well-cooked
or for cooked foods only. These regulations entered into force on January 1, 2016. 123