
2015 Annual Report
121
Risk Assessment
Management and
Research Outcomes
Cosmetics
Management
Appendix
National
Laboratory and
Testing Network
Risk Communication
and Consumer
Protection
International
Cooperation and
Cross-Strait Exchange
Food
Management
Medicinal
Products
Management
Policy and
Organization
Controlled
Drugs
Management
Medical
Devices
Management
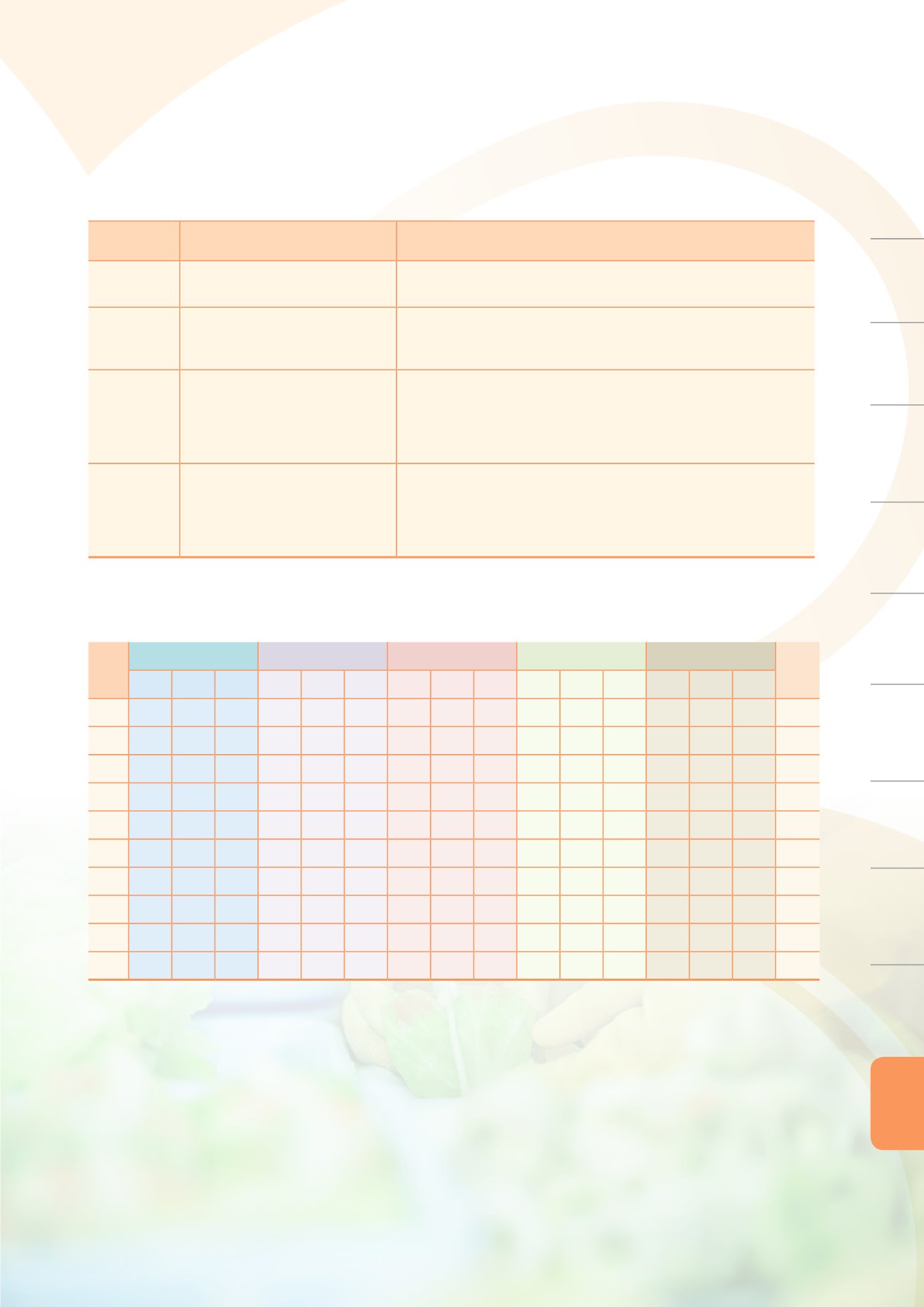
Annex Table 8. Annual statistics on the number of approved drug permit
licenses
Year
Genetic drugs
APIs
NCE
Biological products Orphan drugs
Total
Domestic Imported Subtotal
Domestic Imported Subtotal
Domestic Imported Subtotal
Domestic Imported Subtotal
Domestic Imported Subtotal
2005 369 47 416 8 132 140 18 43 61 0 14 14 2 6 8 639
2006 367 55 422 15 99 114 18 65 83 0 13 13 1 4 5 637
2007 422 32 454 6 115 121 22 47 69 0 16 16 0 0 0 660
2008 383 44 427 13 59 72 18 76 94 1 25 26 2 2 4 623
2009 449 47 496 5 91 96 24 56 80 0 17 17 0 2 2 691
2010 323 41 364 15 69 84 11 77 88 2 14 16 0 0 0 552
2011 220 52 272 20 172 192 17 46 63 1 24 25 0 2 2 554
2012 256 60 316 8 203 211 20 42 62 2 25 27 0 9 9 625
2013 247 51 298 7 105 112 23 14 37 0 1 1 0 3 3 451
2014 263 122 385 24 80 104 28 62 90 1 11 12 1 2 3 594
Date
Regulation / standard name
Summary
23 October
Guidance for Industry: Good
Clinical Practice
Amended the competent authority of Article 2 to the Ministry of
Health and Welfare
2 December
Comparative Testing Standards
for Biotechnological /
Biopharmaceutical Products
This standard was formulated to provide a basis and reference
for businesses when changing manufacturing processes for
biotechnological / biopharmaceutical products
12 December
Priority Review System for New
Drug Applications / Accelerated
Approval Mechanism for New
Drug Applications
Amended the definition of new drugs in Article 7 of the
Pharmaceutical Affairs Act
(new compositions, new
therapeutic compounds, or new method of administration) in
order to accelerate drug research and development as well
as time-to-market
22 December
A List of Adopted ICH Guidelines
A list of the adopted guidelines from the
International Council for
Harmonization of Technical Requirements for Pharmaceuticals for
Human Use (ICH)
was formulated in order to provide a reference
for business owners when preparing technical information for
submission



















