Page 137 - 2023 Taiwan Food and Drug Administration Annual Report
P. 137
7Appendix
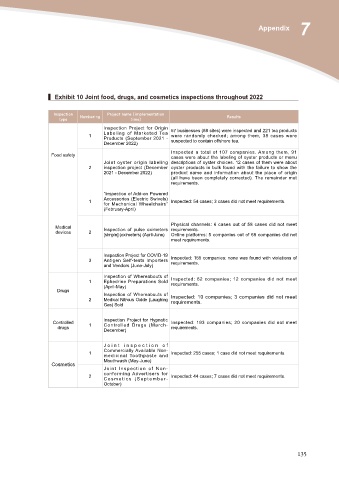
▍ Exhibit 10 Joint food, drugs, and cosmetics inspections throughout 2022
Inspection Numbering Project name (implementation Results
type time)
1 Inspection Project for Origin 57 businesses (88 sites) were inspected and 221 tea products
L a b e l i n g o f M a r k e t e d Te a were randomly checked; among them, 38 cases were
Products (September 2021 - suspected to contain offshore tea.
December 2022)
Food safety Inspected a total of 107 companies. Among them, 91
cases were about the labeling of oyster products or menu
Joint oyster origin labeling descriptions of oyster choices. 12 cases of them were about
2 inspection project (December oyster products in bulk found with the failure to show the
2021 - December 2022) product name and information about the place of origin
(all have been completely corrected). The remainder met
requirements.
“Inspection of Add-on Powered
1 Accessories (Electric Swivels) Inspected: 54 cases; 3 cases did not meet requirements.
for Mechanical Wheelchairs”
(February-April)
Medical Physical channels: 6 cases out of 58 cases did not meet
devices
2 Inspection of pulse oximeters requirements.
[simple] (oximeters) (April-June) Online platforms: 5 companies out of 68 companies did not
meet requirements.
3 Inspection Project for COVID-19 Inspected: 155 companies; none was found with violations of
Antigen Self-tests Importers requirements.
and Vendors (June-July)
1 Inspection of Whereabouts of Inspected: 62 companies; 12 companies did not meet
Ephedrine Preparations Sold requirements.
(April-May)
Drugs
Inspection of Whereabouts of
2 Medical Nitrous Oxide (Laughing Inspected: 10 companies; 3 companies did not meet
Gas) Sold requirements.
Controlled 1 Inspection Project for Hypnotic Inspected: 193 companies; 20 companies did not meet
drugs Controlled Drugs (March- requirements.
December)
Joint inspection of
1 Commercially Available Non- Inspected: 255 cases; 1 case did not meet requirements.
m e d i c i n a l To o t h p a s t e a n d
Cosmetics Mouthwash (May-June)
Joint Inspection of Non-
2 conforming Advertisers for Inspected: 44 cases; 7 cases did not meet requirements.
Cosmetics (September-
October)
135