Page 135 - 2023 Taiwan Food and Drug Administration Annual Report
P. 135
7Appendix
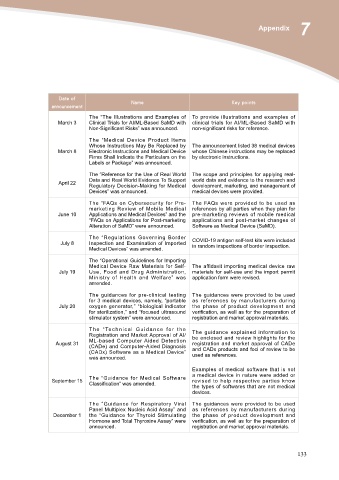
Date of Name Key points
announcement
The “The Illustrations and Examples of To provide illustrations and examples of
March 3 Clinical Trials for AI/ML-Based SaMD with clinical trials for AI/ML-Based SaMD with
Non-Significant Risks” was announced. non-significant risks for reference.
March 8 The “Medical Device Product Items The announcement listed 38 medical devices
Whose Instructions May Be Replaced by whose Chinese instructions may be replaced
Electronic Instructions and Medical Device by electronic instructions.
Firms Shall Indicate the Particulars on the
Labels or Package” was announced.
April 22 The “Reference for the Use of Real World The scope and principles for applying real-
Data and Real World Evidence To Support world data and evidence to the research and
Regulatory Decision-Making for Medical development, marketing, and management of
Devices” was announced. medical devices were provided.
June 10 The “FAQs on Cybersecurity for Pre- The FAQs were provided to be used as
marketing Review of Mobile Medical references by all parties when they plan for
Applications and Medical Devices” and the pre-marketing reviews of mobile medical
“FAQs on Applications for Post-marketing applications and post-market changes of
Alteration of SaMD” were announced. Software as Medical Device (SaMD).
July 8 The “Regulations Governing Border COVID-19 antigen self-test kits were included
Inspection and Examination of Imported in random inspections of border inspection.
Medical Devices” was amended.
July 19 The “Operational Guidelines for Importing The affidavit importing medical device raw
Medical Device Raw Materials for Self- materials for self-use and the import permit
Use, Food and Drug Administration, application form were revised.
Ministry of Health and Welfare” was
amended.
July 20 The guidances for pre-clinical testing The guidances were provided to be used
for 3 medical devices, namely, “portable as references by manufacturers during
oxygen generator,” “biological indicator the phase of product development and
for sterilization,” and “focused ultrasound verification, as well as for the preparation of
stimulator system” were announced. registration and market approval materials.
August 31 T h e “ Te c h n i c a l G u i d a n c e f o r t h e The guidance explained information to
Registration and Market Approval of AI/ be enclosed and review highlights for the
ML-based Computer Aided Detection registration and market approval of CADe
(CADe) and Computer-Aided Diagnosis and CADx products and foci of review to be
(CADx) Software as a Medical Device” used as references.
was announced.
September 15 The “Guidance for Medical Software Examples of medical software that is not
Classification” was amended. a medical device in nature were added or
revised to help respective parties know
the types of softwares that are not medical
devices.
December 1 The “Guidance for Respiratory Viral The guidances were provided to be used
Panel Multiplex Nucleic Acid Assay” and as references by manufacturers during
the “Guidance for Thyroid Stimulating the phase of product development and
Hormone and Total Thyroxine Assay” were verification, as well as for the preparation of
announced. registration and market approval materials.
133